 Found 15 hits of ic50 for monomerid = 50134771
Found 15 hits of ic50 for monomerid = 50134771 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mitogen-activated protein kinase kinase kinase kinase 4
(Homo sapiens (Human)) | BDBM50134771
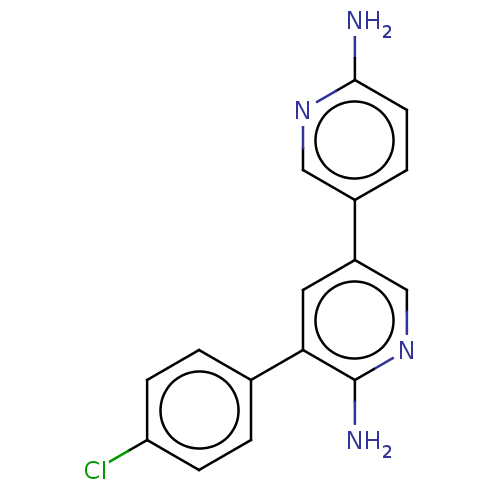
(CHEMBL3754515)Show InChI InChI=1S/C16H13ClN4/c17-13-4-1-10(2-5-13)14-7-12(9-21-16(14)19)11-3-6-15(18)20-8-11/h1-9H,(H2,18,20)(H2,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human MAP4K4 catalytic domain in presence of 10 uM ATP (Km) by FRET assay |
ACS Med Chem Lett 6: 1128-33 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00215
BindingDB Entry DOI: 10.7270/Q2028TC6 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 4
(Homo sapiens (Human)) | BDBM50134771

(CHEMBL3754515)Show InChI InChI=1S/C16H13ClN4/c17-13-4-1-10(2-5-13)14-7-12(9-21-16(14)19)11-3-6-15(18)20-8-11/h1-9H,(H2,18,20)(H2,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human GST-tagged MAP4K4 catalytic domain (1 to 328 residues) expressed in baculovirus expression system pre-incubated for 3... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b00152
BindingDB Entry DOI: 10.7270/Q2TH8RBV |
More data for this
Ligand-Target Pair | |
Misshapen-like kinase 1
(Homo sapiens (Human)) | BDBM50134771

(CHEMBL3754515)Show InChI InChI=1S/C16H13ClN4/c17-13-4-1-10(2-5-13)14-7-12(9-21-16(14)19)11-3-6-15(18)20-8-11/h1-9H,(H2,18,20)(H2,19,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MINK (unknown origin) in presence of ATP (Km) |
ACS Med Chem Lett 6: 1128-33 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00215
BindingDB Entry DOI: 10.7270/Q2028TC6 |
More data for this
Ligand-Target Pair | |
TRAF2 and NCK-interacting protein kinase
(Homo sapiens (Human)) | BDBM50134771

(CHEMBL3754515)Show InChI InChI=1S/C16H13ClN4/c17-13-4-1-10(2-5-13)14-7-12(9-21-16(14)19)11-3-6-15(18)20-8-11/h1-9H,(H2,18,20)(H2,19,21) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of TNIK (unknown origin) in presence of ATP (Km) |
ACS Med Chem Lett 6: 1128-33 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00215
BindingDB Entry DOI: 10.7270/Q2028TC6 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 4
(Homo sapiens (Human)) | BDBM50134771

(CHEMBL3754515)Show InChI InChI=1S/C16H13ClN4/c17-13-4-1-10(2-5-13)14-7-12(9-21-16(14)19)11-3-6-15(18)20-8-11/h1-9H,(H2,18,20)(H2,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 134 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human GST-tagged MAP4K4 catalytic domain (1 to 328 residues) expressed in baculovirus expression system pre-incubated for 3... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b00152
BindingDB Entry DOI: 10.7270/Q2TH8RBV |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 4
(Homo sapiens (Human)) | BDBM50134771

(CHEMBL3754515)Show InChI InChI=1S/C16H13ClN4/c17-13-4-1-10(2-5-13)14-7-12(9-21-16(14)19)11-3-6-15(18)20-8-11/h1-9H,(H2,18,20)(H2,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MAP4K4 transfected in 293 MSR cells assessed as inhibition of phosphorylation of traf2 at ser/thr residue by ELISA |
ACS Med Chem Lett 6: 1128-33 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00215
BindingDB Entry DOI: 10.7270/Q2028TC6 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 4
(Homo sapiens (Human)) | BDBM50134771

(CHEMBL3754515)Show InChI InChI=1S/C16H13ClN4/c17-13-4-1-10(2-5-13)14-7-12(9-21-16(14)19)11-3-6-15(18)20-8-11/h1-9H,(H2,18,20)(H2,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MAP4K4 in human GripTite 293 MSR cells assessed as reduction in TRAF2 Ser/Thr phosphorylation incubated for 1.5 hrs by ELISA |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b00152
BindingDB Entry DOI: 10.7270/Q2TH8RBV |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50134771

(CHEMBL3754515)Show InChI InChI=1S/C16H13ClN4/c17-13-4-1-10(2-5-13)14-7-12(9-21-16(14)19)11-3-6-15(18)20-8-11/h1-9H,(H2,18,20)(H2,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG transfected in HEK293 cells |
ACS Med Chem Lett 6: 1128-33 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00215
BindingDB Entry DOI: 10.7270/Q2028TC6 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50134771

(CHEMBL3754515)Show InChI InChI=1S/C16H13ClN4/c17-13-4-1-10(2-5-13)14-7-12(9-21-16(14)19)11-3-6-15(18)20-8-11/h1-9H,(H2,18,20)(H2,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG expressed in HEK293 cells |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b00152
BindingDB Entry DOI: 10.7270/Q2TH8RBV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50134771

(CHEMBL3754515)Show InChI InChI=1S/C16H13ClN4/c17-13-4-1-10(2-5-13)14-7-12(9-21-16(14)19)11-3-6-15(18)20-8-11/h1-9H,(H2,18,20)(H2,19,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b00152
BindingDB Entry DOI: 10.7270/Q2TH8RBV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50134771

(CHEMBL3754515)Show InChI InChI=1S/C16H13ClN4/c17-13-4-1-10(2-5-13)14-7-12(9-21-16(14)19)11-3-6-15(18)20-8-11/h1-9H,(H2,18,20)(H2,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b00152
BindingDB Entry DOI: 10.7270/Q2TH8RBV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50134771

(CHEMBL3754515)Show InChI InChI=1S/C16H13ClN4/c17-13-4-1-10(2-5-13)14-7-12(9-21-16(14)19)11-3-6-15(18)20-8-11/h1-9H,(H2,18,20)(H2,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b00152
BindingDB Entry DOI: 10.7270/Q2TH8RBV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50134771

(CHEMBL3754515)Show InChI InChI=1S/C16H13ClN4/c17-13-4-1-10(2-5-13)14-7-12(9-21-16(14)19)11-3-6-15(18)20-8-11/h1-9H,(H2,18,20)(H2,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b00152
BindingDB Entry DOI: 10.7270/Q2TH8RBV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50134771

(CHEMBL3754515)Show InChI InChI=1S/C16H13ClN4/c17-13-4-1-10(2-5-13)14-7-12(9-21-16(14)19)11-3-6-15(18)20-8-11/h1-9H,(H2,18,20)(H2,19,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b00152
BindingDB Entry DOI: 10.7270/Q2TH8RBV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50134771

(CHEMBL3754515)Show InChI InChI=1S/C16H13ClN4/c17-13-4-1-10(2-5-13)14-7-12(9-21-16(14)19)11-3-6-15(18)20-8-11/h1-9H,(H2,18,20)(H2,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
ACS Med Chem Lett 6: 1128-33 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00215
BindingDB Entry DOI: 10.7270/Q2028TC6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data