 Found 9 hits of ic50 for monomerid = 50138751
Found 9 hits of ic50 for monomerid = 50138751 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Hormone-sensitive lipase
(Rattus norvegicus (Rat)) | BDBM50138751
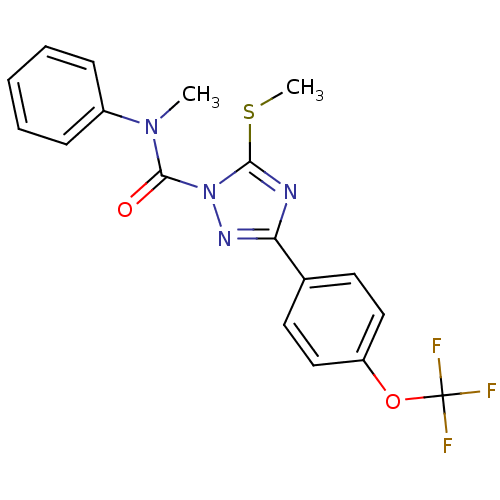
(5-Methylsulfanyl-3-(4-trifluoromethoxy-phenyl)-[1,...)Show SMILES CSc1nc(nn1C(=O)N(C)c1ccccc1)-c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C18H15F3N4O2S/c1-24(13-6-4-3-5-7-13)17(26)25-16(28-2)22-15(23-25)12-8-10-14(11-9-12)27-18(19,20)21/h3-11H,1-2H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.41 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of HSL in Wistar rat isolated fat cells by spectrophotometric assay |
J Med Chem 51: 6478-94 (2008)
Article DOI: 10.1021/jm800718k
BindingDB Entry DOI: 10.7270/Q23778KJ |
More data for this
Ligand-Target Pair | |
Hormone-sensitive lipase
(Homo sapiens (Human)) | BDBM50138751

(5-Methylsulfanyl-3-(4-trifluoromethoxy-phenyl)-[1,...)Show SMILES CSc1nc(nn1C(=O)N(C)c1ccccc1)-c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C18H15F3N4O2S/c1-24(13-6-4-3-5-7-13)17(26)25-16(28-2)22-15(23-25)12-8-10-14(11-9-12)27-18(19,20)21/h3-11H,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibition of hormone sensitive lipase (HSL). |
J Med Chem 47: 400-10 (2004)
Article DOI: 10.1021/jm031004s
BindingDB Entry DOI: 10.7270/Q2ZG6RN1 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50138751

(5-Methylsulfanyl-3-(4-trifluoromethoxy-phenyl)-[1,...)Show SMILES CSc1nc(nn1C(=O)N(C)c1ccccc1)-c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C18H15F3N4O2S/c1-24(13-6-4-3-5-7-13)17(26)25-16(28-2)22-15(23-25)12-8-10-14(11-9-12)27-18(19,20)21/h3-11H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory concentration against butyrylcholinesterase. |
J Med Chem 47: 400-10 (2004)
Article DOI: 10.1021/jm031004s
BindingDB Entry DOI: 10.7270/Q2ZG6RN1 |
More data for this
Ligand-Target Pair | |
Lipoprotein lipase
(Homo sapiens (Human)) | BDBM50138751

(5-Methylsulfanyl-3-(4-trifluoromethoxy-phenyl)-[1,...)Show SMILES CSc1nc(nn1C(=O)N(C)c1ccccc1)-c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C18H15F3N4O2S/c1-24(13-6-4-3-5-7-13)17(26)25-16(28-2)22-15(23-25)12-8-10-14(11-9-12)27-18(19,20)21/h3-11H,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Compound was tested to inhibit lipoprotein lipase (LPL) |
J Med Chem 47: 400-10 (2004)
Article DOI: 10.1021/jm031004s
BindingDB Entry DOI: 10.7270/Q2ZG6RN1 |
More data for this
Ligand-Target Pair | |
Pancreatic triacylglycerol lipase
(Homo sapiens (Human)) | BDBM50138751

(5-Methylsulfanyl-3-(4-trifluoromethoxy-phenyl)-[1,...)Show SMILES CSc1nc(nn1C(=O)N(C)c1ccccc1)-c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C18H15F3N4O2S/c1-24(13-6-4-3-5-7-13)17(26)25-16(28-2)22-15(23-25)12-8-10-14(11-9-12)27-18(19,20)21/h3-11H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Compound was tested to inhibit pancreatic lipase (PL) |
J Med Chem 47: 400-10 (2004)
Article DOI: 10.1021/jm031004s
BindingDB Entry DOI: 10.7270/Q2ZG6RN1 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50138751

(5-Methylsulfanyl-3-(4-trifluoromethoxy-phenyl)-[1,...)Show SMILES CSc1nc(nn1C(=O)N(C)c1ccccc1)-c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C18H15F3N4O2S/c1-24(13-6-4-3-5-7-13)17(26)25-16(28-2)22-15(23-25)12-8-10-14(11-9-12)27-18(19,20)21/h3-11H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory concentration against acetylcholinesterase |
J Med Chem 47: 400-10 (2004)
Article DOI: 10.1021/jm031004s
BindingDB Entry DOI: 10.7270/Q2ZG6RN1 |
More data for this
Ligand-Target Pair | |
Hepatic triacylglycerol lipase
(Homo sapiens (Human)) | BDBM50138751

(5-Methylsulfanyl-3-(4-trifluoromethoxy-phenyl)-[1,...)Show SMILES CSc1nc(nn1C(=O)N(C)c1ccccc1)-c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C18H15F3N4O2S/c1-24(13-6-4-3-5-7-13)17(26)25-16(28-2)22-15(23-25)12-8-10-14(11-9-12)27-18(19,20)21/h3-11H,1-2H3 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Compound was tested to inhibit hepatic lipase (HL) |
J Med Chem 47: 400-10 (2004)
Article DOI: 10.1021/jm031004s
BindingDB Entry DOI: 10.7270/Q2ZG6RN1 |
More data for this
Ligand-Target Pair | |
Hormone-sensitive lipase
(Homo sapiens (Human)) | BDBM50138751

(5-Methylsulfanyl-3-(4-trifluoromethoxy-phenyl)-[1,...)Show SMILES CSc1nc(nn1C(=O)N(C)c1ccccc1)-c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C18H15F3N4O2S/c1-24(13-6-4-3-5-7-13)17(26)25-16(28-2)22-15(23-25)12-8-10-14(11-9-12)27-18(19,20)21/h3-11H,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of HSL (unknown origin) |
J Med Chem 58: 6448-55 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00434
BindingDB Entry DOI: 10.7270/Q2N018BP |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50138751

(5-Methylsulfanyl-3-(4-trifluoromethoxy-phenyl)-[1,...)Show SMILES CSc1nc(nn1C(=O)N(C)c1ccccc1)-c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C18H15F3N4O2S/c1-24(13-6-4-3-5-7-13)17(26)25-16(28-2)22-15(23-25)12-8-10-14(11-9-12)27-18(19,20)21/h3-11H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human AChE |
J Med Chem 58: 6448-55 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00434
BindingDB Entry DOI: 10.7270/Q2N018BP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data