

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM50157605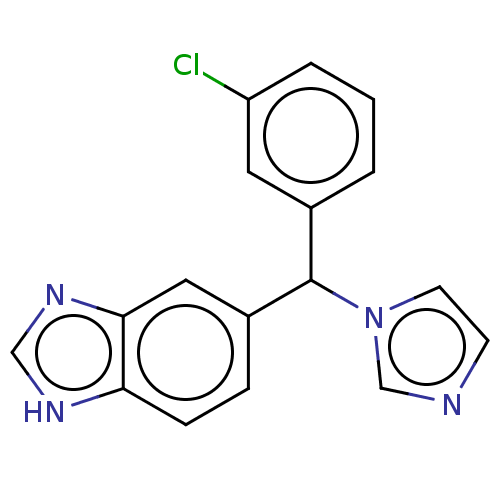 (Liarozole | Liazal | R-75251 | US9963439, Liarozol...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Montana Curated by ChEMBL | Assay Description Inhibition of microsomal fraction of human CYP26B1 expressed in Sf9 cells using 9-cis-RA as substrate preincubated for 5 mins followed by NADPH addit... | J Med Chem 59: 2579-95 (2016) Article DOI: 10.1021/acs.jmedchem.5b01780 BindingDB Entry DOI: 10.7270/Q2J38VG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM50157605 (Liarozole | Liazal | R-75251 | US9963439, Liarozol...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Eighteen compounds were tested as potential inhibitors of CYP26A1 and CYP26B1. The formation of 9-cis-4-OH-RA metabolite was monitored and the percen... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50157605 (Liarozole | Liazal | R-75251 | US9963439, Liarozol...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50157605 (Liarozole | Liazal | R-75251 | US9963439, Liarozol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26A1 (Homo sapiens (Human)) | BDBM50157605 (Liarozole | Liazal | R-75251 | US9963439, Liarozol...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Eighteen compounds were tested as potential inhibitors of CYP26A1 and CYP26B1. The formation of 9-cis-4-OH-RA metabolite was monitored and the percen... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26A1 (Homo sapiens (Human)) | BDBM50157605 (Liarozole | Liazal | R-75251 | US9963439, Liarozol...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Montana Curated by ChEMBL | Assay Description Inhibition of microsomal fraction of human CYP26A1 expressed in Sf9 cells using 9-cis-RA as substrate preincubated for 5 mins followed by NADPH addit... | J Med Chem 59: 2579-95 (2016) Article DOI: 10.1021/acs.jmedchem.5b01780 BindingDB Entry DOI: 10.7270/Q2J38VG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50157605 (Liarozole | Liazal | R-75251 | US9963439, Liarozol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||