 Found 11 hits of ic50 for monomerid = 50308060
Found 11 hits of ic50 for monomerid = 50308060 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50308060
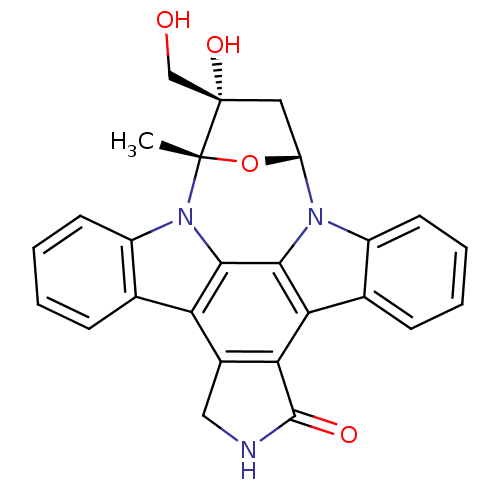
(16-hydroxy-16-(hydroxymethyl)-15-methyl-28-oxa-4,1...)Show SMILES C[C@]12O[C@H](C[C@]1(O)CO)n1c3ccccc3c3c4C(=O)NCc4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C26H21N3O4/c1-25-26(32,12-30)10-18(33-25)28-16-8-4-2-6-13(16)20-21-15(11-27-24(21)31)19-14-7-3-5-9-17(14)29(25)23(19)22(20)28/h2-9,18,30,32H,10-12H2,1H3,(H,27,31)/t18-,25+,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01467
BindingDB Entry DOI: 10.7270/Q24Q801K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50308060

(16-hydroxy-16-(hydroxymethyl)-15-methyl-28-oxa-4,1...)Show SMILES C[C@]12O[C@H](C[C@]1(O)CO)n1c3ccccc3c3c4C(=O)NCc4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C26H21N3O4/c1-25-26(32,12-30)10-18(33-25)28-16-8-4-2-6-13(16)20-21-15(11-27-24(21)31)19-14-7-3-5-9-17(14)29(25)23(19)22(20)28/h2-9,18,30,32H,10-12H2,1H3,(H,27,31)/t18-,25+,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Blocking activity against Beta-2 adrenergic receptor in guinea pig tracheal strips |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50308060

(16-hydroxy-16-(hydroxymethyl)-15-methyl-28-oxa-4,1...)Show SMILES C[C@]12O[C@H](C[C@]1(O)CO)n1c3ccccc3c3c4C(=O)NCc4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C26H21N3O4/c1-25-26(32,12-30)10-18(33-25)28-16-8-4-2-6-13(16)20-21-15(11-27-24(21)31)19-14-7-3-5-9-17(14)29(25)23(19)22(20)28/h2-9,18,30,32H,10-12H2,1H3,(H,27,31)/t18-,25+,26+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 autophosphorylation in human RS4-11 cells after 2 hrs by electrochemiluminescence assay |
Blood 114: 2984-92 (2009)
Article DOI: 10.1182/blood-2009-05-222034
BindingDB Entry DOI: 10.7270/Q2PN95V2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50308060

(16-hydroxy-16-(hydroxymethyl)-15-methyl-28-oxa-4,1...)Show SMILES C[C@]12O[C@H](C[C@]1(O)CO)n1c3ccccc3c3c4C(=O)NCc4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C26H21N3O4/c1-25-26(32,12-30)10-18(33-25)28-16-8-4-2-6-13(16)20-21-15(11-27-24(21)31)19-14-7-3-5-9-17(14)29(25)23(19)22(20)28/h2-9,18,30,32H,10-12H2,1H3,(H,27,31)/t18-,25+,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50308060

(16-hydroxy-16-(hydroxymethyl)-15-methyl-28-oxa-4,1...)Show SMILES C[C@]12O[C@H](C[C@]1(O)CO)n1c3ccccc3c3c4C(=O)NCc4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C26H21N3O4/c1-25-26(32,12-30)10-18(33-25)28-16-8-4-2-6-13(16)20-21-15(11-27-24(21)31)19-14-7-3-5-9-17(14)29(25)23(19)22(20)28/h2-9,18,30,32H,10-12H2,1H3,(H,27,31)/t18-,25+,26+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant His-tagged human FLT3 expressed in baculovirus expression system using peptide substrate incubated for 30 mins in presence ... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112205
BindingDB Entry DOI: 10.7270/Q2VM4H1R |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50308060

(16-hydroxy-16-(hydroxymethyl)-15-methyl-28-oxa-4,1...)Show SMILES C[C@]12O[C@H](C[C@]1(O)CO)n1c3ccccc3c3c4C(=O)NCc4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C26H21N3O4/c1-25-26(32,12-30)10-18(33-25)28-16-8-4-2-6-13(16)20-21-15(11-27-24(21)31)19-14-7-3-5-9-17(14)29(25)23(19)22(20)28/h2-9,18,30,32H,10-12H2,1H3,(H,27,31)/t18-,25+,26+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The inhibition of the FLT3 kinase activity was measured with homogeneous, time-resolved fluorescence (HTRF) assays. Recombinant proteins containing t... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28D00GH |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50308060

(16-hydroxy-16-(hydroxymethyl)-15-methyl-28-oxa-4,1...)Show SMILES C[C@]12O[C@H](C[C@]1(O)CO)n1c3ccccc3c3c4C(=O)NCc4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C26H21N3O4/c1-25-26(32,12-30)10-18(33-25)28-16-8-4-2-6-13(16)20-21-15(11-27-24(21)31)19-14-7-3-5-9-17(14)29(25)23(19)22(20)28/h2-9,18,30,32H,10-12H2,1H3,(H,27,31)/t18-,25+,26+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50308060

(16-hydroxy-16-(hydroxymethyl)-15-methyl-28-oxa-4,1...)Show SMILES C[C@]12O[C@H](C[C@]1(O)CO)n1c3ccccc3c3c4C(=O)NCc4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C26H21N3O4/c1-25-26(32,12-30)10-18(33-25)28-16-8-4-2-6-13(16)20-21-15(11-27-24(21)31)19-14-7-3-5-9-17(14)29(25)23(19)22(20)28/h2-9,18,30,32H,10-12H2,1H3,(H,27,31)/t18-,25+,26+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Blocking activity against Beta-1 adrenergic receptor in spontaneously beating guinea pig atrial preparations |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50308060

(16-hydroxy-16-(hydroxymethyl)-15-methyl-28-oxa-4,1...)Show SMILES C[C@]12O[C@H](C[C@]1(O)CO)n1c3ccccc3c3c4C(=O)NCc4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C26H21N3O4/c1-25-26(32,12-30)10-18(33-25)28-16-8-4-2-6-13(16)20-21-15(11-27-24(21)31)19-14-7-3-5-9-17(14)29(25)23(19)22(20)28/h2-9,18,30,32H,10-12H2,1H3,(H,27,31)/t18-,25+,26+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity towards rat beta adrenergic receptor from lung membranes. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50308060

(16-hydroxy-16-(hydroxymethyl)-15-methyl-28-oxa-4,1...)Show SMILES C[C@]12O[C@H](C[C@]1(O)CO)n1c3ccccc3c3c4C(=O)NCc4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C26H21N3O4/c1-25-26(32,12-30)10-18(33-25)28-16-8-4-2-6-13(16)20-21-15(11-27-24(21)31)19-14-7-3-5-9-17(14)29(25)23(19)22(20)28/h2-9,18,30,32H,10-12H2,1H3,(H,27,31)/t18-,25+,26+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50308060

(16-hydroxy-16-(hydroxymethyl)-15-methyl-28-oxa-4,1...)Show SMILES C[C@]12O[C@H](C[C@]1(O)CO)n1c3ccccc3c3c4C(=O)NCc4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C26H21N3O4/c1-25-26(32,12-30)10-18(33-25)28-16-8-4-2-6-13(16)20-21-15(11-27-24(21)31)19-14-7-3-5-9-17(14)29(25)23(19)22(20)28/h2-9,18,30,32H,10-12H2,1H3,(H,27,31)/t18-,25+,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114054
BindingDB Entry DOI: 10.7270/Q20K2DM8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data