 Found 20 hits of ic50 for monomerid = 50315887
Found 20 hits of ic50 for monomerid = 50315887 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50315887
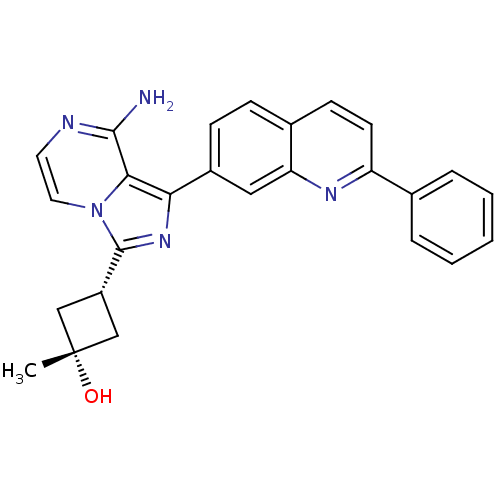
((1s,3s)-3-(8-amino-1-(2-phenylquinolin-7-yl)imidaz...)Show SMILES C[C@@]1(O)C[C@@H](C1)c1nc(-c2ccc3ccc(nc3c2)-c2ccccc2)c2c(N)nccn12 |r,wU:1.1,4.6,wD:1.0,(18.42,-37.26,;17.1,-36.49,;16.31,-37.82,;17.79,-35.11,;16.41,-34.42,;15.72,-35.8,;15.93,-32.97,;16.83,-31.71,;15.91,-30.47,;16.38,-29.01,;15.35,-27.88,;15.81,-26.41,;17.32,-26.08,;17.78,-24.62,;19.27,-24.29,;20.31,-25.42,;19.85,-26.89,;18.35,-27.21,;17.89,-28.68,;21.81,-25.09,;22.85,-26.23,;24.35,-25.9,;24.82,-24.43,;23.77,-23.29,;22.27,-23.63,;14.45,-30.96,;13.11,-30.19,;13.11,-28.65,;11.78,-30.96,;11.78,-32.51,;13.12,-33.28,;14.46,-32.5,)| Show InChI InChI=1S/C26H23N5O/c1-26(32)14-19(15-26)25-30-22(23-24(27)28-11-12-31(23)25)18-8-7-17-9-10-20(29-21(17)13-18)16-5-3-2-4-6-16/h2-13,19,32H,14-15H2,1H3,(H2,27,28)/t19-,26+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Enterprises Limited
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R (unknown origin) using poly-G1 as substrate incubated for 10 mins prior to substrate addition measured after 1 hr by time-resolve... |
Eur J Med Chem 92: 246-56 (2015)
Article DOI: 10.1016/j.ejmech.2014.12.053
BindingDB Entry DOI: 10.7270/Q2639RFV |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50315887

((1s,3s)-3-(8-amino-1-(2-phenylquinolin-7-yl)imidaz...)Show SMILES C[C@@]1(O)C[C@@H](C1)c1nc(-c2ccc3ccc(nc3c2)-c2ccccc2)c2c(N)nccn12 |r,wU:1.1,4.6,wD:1.0,(18.42,-37.26,;17.1,-36.49,;16.31,-37.82,;17.79,-35.11,;16.41,-34.42,;15.72,-35.8,;15.93,-32.97,;16.83,-31.71,;15.91,-30.47,;16.38,-29.01,;15.35,-27.88,;15.81,-26.41,;17.32,-26.08,;17.78,-24.62,;19.27,-24.29,;20.31,-25.42,;19.85,-26.89,;18.35,-27.21,;17.89,-28.68,;21.81,-25.09,;22.85,-26.23,;24.35,-25.9,;24.82,-24.43,;23.77,-23.29,;22.27,-23.63,;14.45,-30.96,;13.11,-30.19,;13.11,-28.65,;11.78,-30.96,;11.78,-32.51,;13.12,-33.28,;14.46,-32.5,)| Show InChI InChI=1S/C26H23N5O/c1-26(32)14-19(15-26)25-30-22(23-24(27)28-11-12-31(23)25)18-8-7-17-9-10-20(29-21(17)13-18)16-5-3-2-4-6-16/h2-13,19,32H,14-15H2,1H3,(H2,27,28)/t19-,26+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of full length IGF-1 receptor (unknown origin) autophosphorylation transfected in HEK293 cells pretreated for 60 mins followed by IGF-1 st... |
Bioorg Med Chem Lett 26: 2057-64 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.075
BindingDB Entry DOI: 10.7270/Q29K4D4J |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50315887

((1s,3s)-3-(8-amino-1-(2-phenylquinolin-7-yl)imidaz...)Show SMILES C[C@@]1(O)C[C@@H](C1)c1nc(-c2ccc3ccc(nc3c2)-c2ccccc2)c2c(N)nccn12 |r,wU:1.1,4.6,wD:1.0,(18.42,-37.26,;17.1,-36.49,;16.31,-37.82,;17.79,-35.11,;16.41,-34.42,;15.72,-35.8,;15.93,-32.97,;16.83,-31.71,;15.91,-30.47,;16.38,-29.01,;15.35,-27.88,;15.81,-26.41,;17.32,-26.08,;17.78,-24.62,;19.27,-24.29,;20.31,-25.42,;19.85,-26.89,;18.35,-27.21,;17.89,-28.68,;21.81,-25.09,;22.85,-26.23,;24.35,-25.9,;24.82,-24.43,;23.77,-23.29,;22.27,-23.63,;14.45,-30.96,;13.11,-30.19,;13.11,-28.65,;11.78,-30.96,;11.78,-32.51,;13.12,-33.28,;14.46,-32.5,)| Show InChI InChI=1S/C26H23N5O/c1-26(32)14-19(15-26)25-30-22(23-24(27)28-11-12-31(23)25)18-8-7-17-9-10-20(29-21(17)13-18)16-5-3-2-4-6-16/h2-13,19,32H,14-15H2,1H3,(H2,27,28)/t19-,26+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of full length IGF-1 receptor (unknown origin) transfected in Ba/F3 cells assessed as cell proliferation |
Bioorg Med Chem Lett 26: 2057-64 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.075
BindingDB Entry DOI: 10.7270/Q29K4D4J |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50315887

((1s,3s)-3-(8-amino-1-(2-phenylquinolin-7-yl)imidaz...)Show SMILES C[C@@]1(O)C[C@@H](C1)c1nc(-c2ccc3ccc(nc3c2)-c2ccccc2)c2c(N)nccn12 |r,wU:1.1,4.6,wD:1.0,(18.42,-37.26,;17.1,-36.49,;16.31,-37.82,;17.79,-35.11,;16.41,-34.42,;15.72,-35.8,;15.93,-32.97,;16.83,-31.71,;15.91,-30.47,;16.38,-29.01,;15.35,-27.88,;15.81,-26.41,;17.32,-26.08,;17.78,-24.62,;19.27,-24.29,;20.31,-25.42,;19.85,-26.89,;18.35,-27.21,;17.89,-28.68,;21.81,-25.09,;22.85,-26.23,;24.35,-25.9,;24.82,-24.43,;23.77,-23.29,;22.27,-23.63,;14.45,-30.96,;13.11,-30.19,;13.11,-28.65,;11.78,-30.96,;11.78,-32.51,;13.12,-33.28,;14.46,-32.5,)| Show InChI InChI=1S/C26H23N5O/c1-26(32)14-19(15-26)25-30-22(23-24(27)28-11-12-31(23)25)18-8-7-17-9-10-20(29-21(17)13-18)16-5-3-2-4-6-16/h2-13,19,32H,14-15H2,1H3,(H2,27,28)/t19-,26+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, LLC
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged IGF-1R catalytic domain (unknown origin) using omnia Y peptide-12 as substrate assessed as inhibition of substrate phosphory... |
ACS Med Chem Lett 4: 627-31 (2013)
Article DOI: 10.1021/ml400160a
BindingDB Entry DOI: 10.7270/Q2S75HR4 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50315887

((1s,3s)-3-(8-amino-1-(2-phenylquinolin-7-yl)imidaz...)Show SMILES C[C@@]1(O)C[C@@H](C1)c1nc(-c2ccc3ccc(nc3c2)-c2ccccc2)c2c(N)nccn12 |r,wU:1.1,4.6,wD:1.0,(18.42,-37.26,;17.1,-36.49,;16.31,-37.82,;17.79,-35.11,;16.41,-34.42,;15.72,-35.8,;15.93,-32.97,;16.83,-31.71,;15.91,-30.47,;16.38,-29.01,;15.35,-27.88,;15.81,-26.41,;17.32,-26.08,;17.78,-24.62,;19.27,-24.29,;20.31,-25.42,;19.85,-26.89,;18.35,-27.21,;17.89,-28.68,;21.81,-25.09,;22.85,-26.23,;24.35,-25.9,;24.82,-24.43,;23.77,-23.29,;22.27,-23.63,;14.45,-30.96,;13.11,-30.19,;13.11,-28.65,;11.78,-30.96,;11.78,-32.51,;13.12,-33.28,;14.46,-32.5,)| Show InChI InChI=1S/C26H23N5O/c1-26(32)14-19(15-26)25-30-22(23-24(27)28-11-12-31(23)25)18-8-7-17-9-10-20(29-21(17)13-18)16-5-3-2-4-6-16/h2-13,19,32H,14-15H2,1H3,(H2,27,28)/t19-,26+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, LLC
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged IGF-1R catalytic domain (unknown origin) using omnia Y peptide-12 as substrate assessed as inhibition of substrate phosphory... |
ACS Med Chem Lett 4: 627-31 (2013)
Article DOI: 10.1021/ml400160a
BindingDB Entry DOI: 10.7270/Q2S75HR4 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50315887

((1s,3s)-3-(8-amino-1-(2-phenylquinolin-7-yl)imidaz...)Show SMILES C[C@@]1(O)C[C@@H](C1)c1nc(-c2ccc3ccc(nc3c2)-c2ccccc2)c2c(N)nccn12 |r,wU:1.1,4.6,wD:1.0,(18.42,-37.26,;17.1,-36.49,;16.31,-37.82,;17.79,-35.11,;16.41,-34.42,;15.72,-35.8,;15.93,-32.97,;16.83,-31.71,;15.91,-30.47,;16.38,-29.01,;15.35,-27.88,;15.81,-26.41,;17.32,-26.08,;17.78,-24.62,;19.27,-24.29,;20.31,-25.42,;19.85,-26.89,;18.35,-27.21,;17.89,-28.68,;21.81,-25.09,;22.85,-26.23,;24.35,-25.9,;24.82,-24.43,;23.77,-23.29,;22.27,-23.63,;14.45,-30.96,;13.11,-30.19,;13.11,-28.65,;11.78,-30.96,;11.78,-32.51,;13.12,-33.28,;14.46,-32.5,)| Show InChI InChI=1S/C26H23N5O/c1-26(32)14-19(15-26)25-30-22(23-24(27)28-11-12-31(23)25)18-8-7-17-9-10-20(29-21(17)13-18)16-5-3-2-4-6-16/h2-13,19,32H,14-15H2,1H3,(H2,27,28)/t19-,26+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of IGF-1 receptor (unknown origin) in presence of [gamma33P]ATP |
Bioorg Med Chem Lett 26: 2057-64 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.075
BindingDB Entry DOI: 10.7270/Q29K4D4J |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50315887

((1s,3s)-3-(8-amino-1-(2-phenylquinolin-7-yl)imidaz...)Show SMILES C[C@@]1(O)C[C@@H](C1)c1nc(-c2ccc3ccc(nc3c2)-c2ccccc2)c2c(N)nccn12 |r,wU:1.1,4.6,wD:1.0,(18.42,-37.26,;17.1,-36.49,;16.31,-37.82,;17.79,-35.11,;16.41,-34.42,;15.72,-35.8,;15.93,-32.97,;16.83,-31.71,;15.91,-30.47,;16.38,-29.01,;15.35,-27.88,;15.81,-26.41,;17.32,-26.08,;17.78,-24.62,;19.27,-24.29,;20.31,-25.42,;19.85,-26.89,;18.35,-27.21,;17.89,-28.68,;21.81,-25.09,;22.85,-26.23,;24.35,-25.9,;24.82,-24.43,;23.77,-23.29,;22.27,-23.63,;14.45,-30.96,;13.11,-30.19,;13.11,-28.65,;11.78,-30.96,;11.78,-32.51,;13.12,-33.28,;14.46,-32.5,)| Show InChI InChI=1S/C26H23N5O/c1-26(32)14-19(15-26)25-30-22(23-24(27)28-11-12-31(23)25)18-8-7-17-9-10-20(29-21(17)13-18)16-5-3-2-4-6-16/h2-13,19,32H,14-15H2,1H3,(H2,27,28)/t19-,26+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of full length insulin receptor (unknown origin) autophosphorylation transfected in HEK293 cells pretreated for 60 mins followed by IGF-1 ... |
Bioorg Med Chem Lett 26: 2057-64 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.075
BindingDB Entry DOI: 10.7270/Q29K4D4J |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50315887

((1s,3s)-3-(8-amino-1-(2-phenylquinolin-7-yl)imidaz...)Show SMILES C[C@@]1(O)C[C@@H](C1)c1nc(-c2ccc3ccc(nc3c2)-c2ccccc2)c2c(N)nccn12 |r,wU:1.1,4.6,wD:1.0,(18.42,-37.26,;17.1,-36.49,;16.31,-37.82,;17.79,-35.11,;16.41,-34.42,;15.72,-35.8,;15.93,-32.97,;16.83,-31.71,;15.91,-30.47,;16.38,-29.01,;15.35,-27.88,;15.81,-26.41,;17.32,-26.08,;17.78,-24.62,;19.27,-24.29,;20.31,-25.42,;19.85,-26.89,;18.35,-27.21,;17.89,-28.68,;21.81,-25.09,;22.85,-26.23,;24.35,-25.9,;24.82,-24.43,;23.77,-23.29,;22.27,-23.63,;14.45,-30.96,;13.11,-30.19,;13.11,-28.65,;11.78,-30.96,;11.78,-32.51,;13.12,-33.28,;14.46,-32.5,)| Show InChI InChI=1S/C26H23N5O/c1-26(32)14-19(15-26)25-30-22(23-24(27)28-11-12-31(23)25)18-8-7-17-9-10-20(29-21(17)13-18)16-5-3-2-4-6-16/h2-13,19,32H,14-15H2,1H3,(H2,27,28)/t19-,26+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IGF1R expressed in Sf21 cells by time resolved fluorescence assay |
Bioorg Med Chem Lett 20: 2452-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.015
BindingDB Entry DOI: 10.7270/Q2M908VT |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50315887

((1s,3s)-3-(8-amino-1-(2-phenylquinolin-7-yl)imidaz...)Show SMILES C[C@@]1(O)C[C@@H](C1)c1nc(-c2ccc3ccc(nc3c2)-c2ccccc2)c2c(N)nccn12 |r,wU:1.1,4.6,wD:1.0,(18.42,-37.26,;17.1,-36.49,;16.31,-37.82,;17.79,-35.11,;16.41,-34.42,;15.72,-35.8,;15.93,-32.97,;16.83,-31.71,;15.91,-30.47,;16.38,-29.01,;15.35,-27.88,;15.81,-26.41,;17.32,-26.08,;17.78,-24.62,;19.27,-24.29,;20.31,-25.42,;19.85,-26.89,;18.35,-27.21,;17.89,-28.68,;21.81,-25.09,;22.85,-26.23,;24.35,-25.9,;24.82,-24.43,;23.77,-23.29,;22.27,-23.63,;14.45,-30.96,;13.11,-30.19,;13.11,-28.65,;11.78,-30.96,;11.78,-32.51,;13.12,-33.28,;14.46,-32.5,)| Show InChI InChI=1S/C26H23N5O/c1-26(32)14-19(15-26)25-30-22(23-24(27)28-11-12-31(23)25)18-8-7-17-9-10-20(29-21(17)13-18)16-5-3-2-4-6-16/h2-13,19,32H,14-15H2,1H3,(H2,27,28)/t19-,26+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Vichem Chemie Research Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant INSR (unknown origin) using fluorescent dye-labelled KKSRGDYMTMQIG peptide peptide as substrate after 1 hr by IMAP assay |
ACS Med Chem Lett 5: 298-303 (2014)
Article DOI: 10.1021/ml4003309
BindingDB Entry DOI: 10.7270/Q2HD7X6X |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50315887

((1s,3s)-3-(8-amino-1-(2-phenylquinolin-7-yl)imidaz...)Show SMILES C[C@@]1(O)C[C@@H](C1)c1nc(-c2ccc3ccc(nc3c2)-c2ccccc2)c2c(N)nccn12 |r,wU:1.1,4.6,wD:1.0,(18.42,-37.26,;17.1,-36.49,;16.31,-37.82,;17.79,-35.11,;16.41,-34.42,;15.72,-35.8,;15.93,-32.97,;16.83,-31.71,;15.91,-30.47,;16.38,-29.01,;15.35,-27.88,;15.81,-26.41,;17.32,-26.08,;17.78,-24.62,;19.27,-24.29,;20.31,-25.42,;19.85,-26.89,;18.35,-27.21,;17.89,-28.68,;21.81,-25.09,;22.85,-26.23,;24.35,-25.9,;24.82,-24.43,;23.77,-23.29,;22.27,-23.63,;14.45,-30.96,;13.11,-30.19,;13.11,-28.65,;11.78,-30.96,;11.78,-32.51,;13.12,-33.28,;14.46,-32.5,)| Show InChI InChI=1S/C26H23N5O/c1-26(32)14-19(15-26)25-30-22(23-24(27)28-11-12-31(23)25)18-8-7-17-9-10-20(29-21(17)13-18)16-5-3-2-4-6-16/h2-13,19,32H,14-15H2,1H3,(H2,27,28)/t19-,26+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, LLC
Curated by ChEMBL
| Assay Description
Inhibition of full length human IGF-1R overexpressed in mouse NIH 3T3 cells assessed as IGF1-induced protein phosphorylation incubated for 2 hrs prio... |
ACS Med Chem Lett 4: 627-31 (2013)
Article DOI: 10.1021/ml400160a
BindingDB Entry DOI: 10.7270/Q2S75HR4 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50315887

((1s,3s)-3-(8-amino-1-(2-phenylquinolin-7-yl)imidaz...)Show SMILES C[C@@]1(O)C[C@@H](C1)c1nc(-c2ccc3ccc(nc3c2)-c2ccccc2)c2c(N)nccn12 |r,wU:1.1,4.6,wD:1.0,(18.42,-37.26,;17.1,-36.49,;16.31,-37.82,;17.79,-35.11,;16.41,-34.42,;15.72,-35.8,;15.93,-32.97,;16.83,-31.71,;15.91,-30.47,;16.38,-29.01,;15.35,-27.88,;15.81,-26.41,;17.32,-26.08,;17.78,-24.62,;19.27,-24.29,;20.31,-25.42,;19.85,-26.89,;18.35,-27.21,;17.89,-28.68,;21.81,-25.09,;22.85,-26.23,;24.35,-25.9,;24.82,-24.43,;23.77,-23.29,;22.27,-23.63,;14.45,-30.96,;13.11,-30.19,;13.11,-28.65,;11.78,-30.96,;11.78,-32.51,;13.12,-33.28,;14.46,-32.5,)| Show InChI InChI=1S/C26H23N5O/c1-26(32)14-19(15-26)25-30-22(23-24(27)28-11-12-31(23)25)18-8-7-17-9-10-20(29-21(17)13-18)16-5-3-2-4-6-16/h2-13,19,32H,14-15H2,1H3,(H2,27,28)/t19-,26+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IGF1R expressed in mouse 3T3 cells by ELISA based assay |
Bioorg Med Chem Lett 21: 1176-80 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.094
BindingDB Entry DOI: 10.7270/Q2J103DJ |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50315887

((1s,3s)-3-(8-amino-1-(2-phenylquinolin-7-yl)imidaz...)Show SMILES C[C@@]1(O)C[C@@H](C1)c1nc(-c2ccc3ccc(nc3c2)-c2ccccc2)c2c(N)nccn12 |r,wU:1.1,4.6,wD:1.0,(18.42,-37.26,;17.1,-36.49,;16.31,-37.82,;17.79,-35.11,;16.41,-34.42,;15.72,-35.8,;15.93,-32.97,;16.83,-31.71,;15.91,-30.47,;16.38,-29.01,;15.35,-27.88,;15.81,-26.41,;17.32,-26.08,;17.78,-24.62,;19.27,-24.29,;20.31,-25.42,;19.85,-26.89,;18.35,-27.21,;17.89,-28.68,;21.81,-25.09,;22.85,-26.23,;24.35,-25.9,;24.82,-24.43,;23.77,-23.29,;22.27,-23.63,;14.45,-30.96,;13.11,-30.19,;13.11,-28.65,;11.78,-30.96,;11.78,-32.51,;13.12,-33.28,;14.46,-32.5,)| Show InChI InChI=1S/C26H23N5O/c1-26(32)14-19(15-26)25-30-22(23-24(27)28-11-12-31(23)25)18-8-7-17-9-10-20(29-21(17)13-18)16-5-3-2-4-6-16/h2-13,19,32H,14-15H2,1H3,(H2,27,28)/t19-,26+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of insulin receptor (unknown origin) in presence of [gamma33P]ATP |
Bioorg Med Chem Lett 26: 2057-64 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.075
BindingDB Entry DOI: 10.7270/Q29K4D4J |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50315887

((1s,3s)-3-(8-amino-1-(2-phenylquinolin-7-yl)imidaz...)Show SMILES C[C@@]1(O)C[C@@H](C1)c1nc(-c2ccc3ccc(nc3c2)-c2ccccc2)c2c(N)nccn12 |r,wU:1.1,4.6,wD:1.0,(18.42,-37.26,;17.1,-36.49,;16.31,-37.82,;17.79,-35.11,;16.41,-34.42,;15.72,-35.8,;15.93,-32.97,;16.83,-31.71,;15.91,-30.47,;16.38,-29.01,;15.35,-27.88,;15.81,-26.41,;17.32,-26.08,;17.78,-24.62,;19.27,-24.29,;20.31,-25.42,;19.85,-26.89,;18.35,-27.21,;17.89,-28.68,;21.81,-25.09,;22.85,-26.23,;24.35,-25.9,;24.82,-24.43,;23.77,-23.29,;22.27,-23.63,;14.45,-30.96,;13.11,-30.19,;13.11,-28.65,;11.78,-30.96,;11.78,-32.51,;13.12,-33.28,;14.46,-32.5,)| Show InChI InChI=1S/C26H23N5O/c1-26(32)14-19(15-26)25-30-22(23-24(27)28-11-12-31(23)25)18-8-7-17-9-10-20(29-21(17)13-18)16-5-3-2-4-6-16/h2-13,19,32H,14-15H2,1H3,(H2,27,28)/t19-,26+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Compound was tested for the biological activity at the Beta-1 adrenergic receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50315887

((1s,3s)-3-(8-amino-1-(2-phenylquinolin-7-yl)imidaz...)Show SMILES C[C@@]1(O)C[C@@H](C1)c1nc(-c2ccc3ccc(nc3c2)-c2ccccc2)c2c(N)nccn12 |r,wU:1.1,4.6,wD:1.0,(18.42,-37.26,;17.1,-36.49,;16.31,-37.82,;17.79,-35.11,;16.41,-34.42,;15.72,-35.8,;15.93,-32.97,;16.83,-31.71,;15.91,-30.47,;16.38,-29.01,;15.35,-27.88,;15.81,-26.41,;17.32,-26.08,;17.78,-24.62,;19.27,-24.29,;20.31,-25.42,;19.85,-26.89,;18.35,-27.21,;17.89,-28.68,;21.81,-25.09,;22.85,-26.23,;24.35,-25.9,;24.82,-24.43,;23.77,-23.29,;22.27,-23.63,;14.45,-30.96,;13.11,-30.19,;13.11,-28.65,;11.78,-30.96,;11.78,-32.51,;13.12,-33.28,;14.46,-32.5,)| Show InChI InChI=1S/C26H23N5O/c1-26(32)14-19(15-26)25-30-22(23-24(27)28-11-12-31(23)25)18-8-7-17-9-10-20(29-21(17)13-18)16-5-3-2-4-6-16/h2-13,19,32H,14-15H2,1H3,(H2,27,28)/t19-,26+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50315887

((1s,3s)-3-(8-amino-1-(2-phenylquinolin-7-yl)imidaz...)Show SMILES C[C@@]1(O)C[C@@H](C1)c1nc(-c2ccc3ccc(nc3c2)-c2ccccc2)c2c(N)nccn12 |r,wU:1.1,4.6,wD:1.0,(18.42,-37.26,;17.1,-36.49,;16.31,-37.82,;17.79,-35.11,;16.41,-34.42,;15.72,-35.8,;15.93,-32.97,;16.83,-31.71,;15.91,-30.47,;16.38,-29.01,;15.35,-27.88,;15.81,-26.41,;17.32,-26.08,;17.78,-24.62,;19.27,-24.29,;20.31,-25.42,;19.85,-26.89,;18.35,-27.21,;17.89,-28.68,;21.81,-25.09,;22.85,-26.23,;24.35,-25.9,;24.82,-24.43,;23.77,-23.29,;22.27,-23.63,;14.45,-30.96,;13.11,-30.19,;13.11,-28.65,;11.78,-30.96,;11.78,-32.51,;13.12,-33.28,;14.46,-32.5,)| Show InChI InChI=1S/C26H23N5O/c1-26(32)14-19(15-26)25-30-22(23-24(27)28-11-12-31(23)25)18-8-7-17-9-10-20(29-21(17)13-18)16-5-3-2-4-6-16/h2-13,19,32H,14-15H2,1H3,(H2,27,28)/t19-,26+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Activity at beta-1 adrenergic receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50315887

((1s,3s)-3-(8-amino-1-(2-phenylquinolin-7-yl)imidaz...)Show SMILES C[C@@]1(O)C[C@@H](C1)c1nc(-c2ccc3ccc(nc3c2)-c2ccccc2)c2c(N)nccn12 |r,wU:1.1,4.6,wD:1.0,(18.42,-37.26,;17.1,-36.49,;16.31,-37.82,;17.79,-35.11,;16.41,-34.42,;15.72,-35.8,;15.93,-32.97,;16.83,-31.71,;15.91,-30.47,;16.38,-29.01,;15.35,-27.88,;15.81,-26.41,;17.32,-26.08,;17.78,-24.62,;19.27,-24.29,;20.31,-25.42,;19.85,-26.89,;18.35,-27.21,;17.89,-28.68,;21.81,-25.09,;22.85,-26.23,;24.35,-25.9,;24.82,-24.43,;23.77,-23.29,;22.27,-23.63,;14.45,-30.96,;13.11,-30.19,;13.11,-28.65,;11.78,-30.96,;11.78,-32.51,;13.12,-33.28,;14.46,-32.5,)| Show InChI InChI=1S/C26H23N5O/c1-26(32)14-19(15-26)25-30-22(23-24(27)28-11-12-31(23)25)18-8-7-17-9-10-20(29-21(17)13-18)16-5-3-2-4-6-16/h2-13,19,32H,14-15H2,1H3,(H2,27,28)/t19-,26+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of full length insulin receptor (unknown origin) transfected in Ba/F3 cells assessed as cell proliferation |
Bioorg Med Chem Lett 26: 2057-64 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.075
BindingDB Entry DOI: 10.7270/Q29K4D4J |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50315887

((1s,3s)-3-(8-amino-1-(2-phenylquinolin-7-yl)imidaz...)Show SMILES C[C@@]1(O)C[C@@H](C1)c1nc(-c2ccc3ccc(nc3c2)-c2ccccc2)c2c(N)nccn12 |r,wU:1.1,4.6,wD:1.0,(18.42,-37.26,;17.1,-36.49,;16.31,-37.82,;17.79,-35.11,;16.41,-34.42,;15.72,-35.8,;15.93,-32.97,;16.83,-31.71,;15.91,-30.47,;16.38,-29.01,;15.35,-27.88,;15.81,-26.41,;17.32,-26.08,;17.78,-24.62,;19.27,-24.29,;20.31,-25.42,;19.85,-26.89,;18.35,-27.21,;17.89,-28.68,;21.81,-25.09,;22.85,-26.23,;24.35,-25.9,;24.82,-24.43,;23.77,-23.29,;22.27,-23.63,;14.45,-30.96,;13.11,-30.19,;13.11,-28.65,;11.78,-30.96,;11.78,-32.51,;13.12,-33.28,;14.46,-32.5,)| Show InChI InChI=1S/C26H23N5O/c1-26(32)14-19(15-26)25-30-22(23-24(27)28-11-12-31(23)25)18-8-7-17-9-10-20(29-21(17)13-18)16-5-3-2-4-6-16/h2-13,19,32H,14-15H2,1H3,(H2,27,28)/t19-,26+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of EGFR intracellular phosphorylation in human A431 cells by ELISA |
Bioorg Med Chem Lett 20: 2452-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.015
BindingDB Entry DOI: 10.7270/Q2M908VT |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50315887

((1s,3s)-3-(8-amino-1-(2-phenylquinolin-7-yl)imidaz...)Show SMILES C[C@@]1(O)C[C@@H](C1)c1nc(-c2ccc3ccc(nc3c2)-c2ccccc2)c2c(N)nccn12 |r,wU:1.1,4.6,wD:1.0,(18.42,-37.26,;17.1,-36.49,;16.31,-37.82,;17.79,-35.11,;16.41,-34.42,;15.72,-35.8,;15.93,-32.97,;16.83,-31.71,;15.91,-30.47,;16.38,-29.01,;15.35,-27.88,;15.81,-26.41,;17.32,-26.08,;17.78,-24.62,;19.27,-24.29,;20.31,-25.42,;19.85,-26.89,;18.35,-27.21,;17.89,-28.68,;21.81,-25.09,;22.85,-26.23,;24.35,-25.9,;24.82,-24.43,;23.77,-23.29,;22.27,-23.63,;14.45,-30.96,;13.11,-30.19,;13.11,-28.65,;11.78,-30.96,;11.78,-32.51,;13.12,-33.28,;14.46,-32.5,)| Show InChI InChI=1S/C26H23N5O/c1-26(32)14-19(15-26)25-30-22(23-24(27)28-11-12-31(23)25)18-8-7-17-9-10-20(29-21(17)13-18)16-5-3-2-4-6-16/h2-13,19,32H,14-15H2,1H3,(H2,27,28)/t19-,26+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 by time resolved fluorescence assay |
Bioorg Med Chem Lett 20: 2452-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.015
BindingDB Entry DOI: 10.7270/Q2M908VT |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50315887

((1s,3s)-3-(8-amino-1-(2-phenylquinolin-7-yl)imidaz...)Show SMILES C[C@@]1(O)C[C@@H](C1)c1nc(-c2ccc3ccc(nc3c2)-c2ccccc2)c2c(N)nccn12 |r,wU:1.1,4.6,wD:1.0,(18.42,-37.26,;17.1,-36.49,;16.31,-37.82,;17.79,-35.11,;16.41,-34.42,;15.72,-35.8,;15.93,-32.97,;16.83,-31.71,;15.91,-30.47,;16.38,-29.01,;15.35,-27.88,;15.81,-26.41,;17.32,-26.08,;17.78,-24.62,;19.27,-24.29,;20.31,-25.42,;19.85,-26.89,;18.35,-27.21,;17.89,-28.68,;21.81,-25.09,;22.85,-26.23,;24.35,-25.9,;24.82,-24.43,;23.77,-23.29,;22.27,-23.63,;14.45,-30.96,;13.11,-30.19,;13.11,-28.65,;11.78,-30.96,;11.78,-32.51,;13.12,-33.28,;14.46,-32.5,)| Show InChI InChI=1S/C26H23N5O/c1-26(32)14-19(15-26)25-30-22(23-24(27)28-11-12-31(23)25)18-8-7-17-9-10-20(29-21(17)13-18)16-5-3-2-4-6-16/h2-13,19,32H,14-15H2,1H3,(H2,27,28)/t19-,26+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vichem Chemie Research Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant EGFR (unknown origin) using poly-GT peptide as substrate by Transcreener assay |
ACS Med Chem Lett 5: 298-303 (2014)
Article DOI: 10.1021/ml4003309
BindingDB Entry DOI: 10.7270/Q2HD7X6X |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50315887

((1s,3s)-3-(8-amino-1-(2-phenylquinolin-7-yl)imidaz...)Show SMILES C[C@@]1(O)C[C@@H](C1)c1nc(-c2ccc3ccc(nc3c2)-c2ccccc2)c2c(N)nccn12 |r,wU:1.1,4.6,wD:1.0,(18.42,-37.26,;17.1,-36.49,;16.31,-37.82,;17.79,-35.11,;16.41,-34.42,;15.72,-35.8,;15.93,-32.97,;16.83,-31.71,;15.91,-30.47,;16.38,-29.01,;15.35,-27.88,;15.81,-26.41,;17.32,-26.08,;17.78,-24.62,;19.27,-24.29,;20.31,-25.42,;19.85,-26.89,;18.35,-27.21,;17.89,-28.68,;21.81,-25.09,;22.85,-26.23,;24.35,-25.9,;24.82,-24.43,;23.77,-23.29,;22.27,-23.63,;14.45,-30.96,;13.11,-30.19,;13.11,-28.65,;11.78,-30.96,;11.78,-32.51,;13.12,-33.28,;14.46,-32.5,)| Show InChI InChI=1S/C26H23N5O/c1-26(32)14-19(15-26)25-30-22(23-24(27)28-11-12-31(23)25)18-8-7-17-9-10-20(29-21(17)13-18)16-5-3-2-4-6-16/h2-13,19,32H,14-15H2,1H3,(H2,27,28)/t19-,26+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vichem Chemie Research Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant c-MET (unknown origin) using poly-AEKY peptide as substrate after 60 mins by ADPGlo assay |
ACS Med Chem Lett 5: 298-303 (2014)
Article DOI: 10.1021/ml4003309
BindingDB Entry DOI: 10.7270/Q2HD7X6X |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data