

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2X purinoceptor 2 (RAT) | BDBM50336786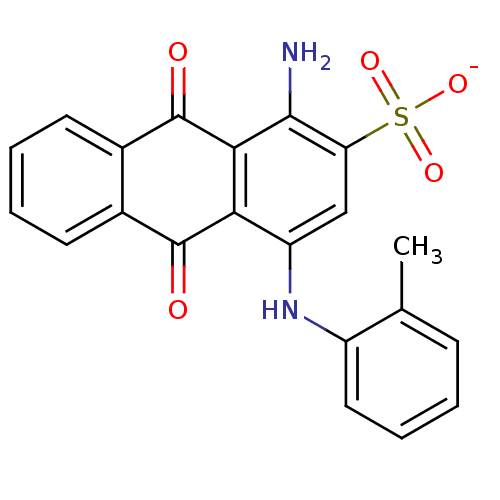 (CHEMBL256864 | sodium 1-amino-9,10-dioxo-4-(o-toly...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Antagonist activity against rat P2X2 receptor expressed in Xenopus laevis oocyte assessed as inhibition of alpha, beta-meATP-induced inward current b... | J Med Chem 54: 817-30 (2012) Article DOI: 10.1021/jm1012193 BindingDB Entry DOI: 10.7270/Q2VH5PTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 4 (Homo sapiens (Human)) | BDBM50336786 (CHEMBL256864 | sodium 1-amino-9,10-dioxo-4-(o-toly...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Antagonist activity at human P2Y4 receptor transfected in human 1321N1 cells assessed as inhibition of UTP-activated intracellular calcium mobilizati... | J Med Chem 60: 3020-3038 (2017) Article DOI: 10.1021/acs.jmedchem.7b00030 BindingDB Entry DOI: 10.7270/Q2G73H15 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (RAT) | BDBM50336786 (CHEMBL256864 | sodium 1-amino-9,10-dioxo-4-(o-toly...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Antagonist activity against rat P2X4 receptor expressed in Xenopus laevis oocyte assessed as inhibition of alpha, beta-meATP-induced inward current b... | J Med Chem 54: 817-30 (2012) Article DOI: 10.1021/jm1012193 BindingDB Entry DOI: 10.7270/Q2VH5PTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Mus musculus) | BDBM50336786 (CHEMBL256864 | sodium 1-amino-9,10-dioxo-4-(o-toly...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y2 receptor in mouse NG108-15 cells assessed as inhibition of UTP-induced calcium mobilization | Bioorg Med Chem Lett 18: 223-7 (2008) Article DOI: 10.1016/j.bmcl.2007.10.082 BindingDB Entry DOI: 10.7270/Q2RX9CXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50336786 (CHEMBL256864 | sodium 1-amino-9,10-dioxo-4-(o-toly...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Antagonist activity at human recombinant P2Y2 receptor in 1321N1 cells assessed as inhibition of UTP-induced calcium mobilization | Bioorg Med Chem Lett 18: 223-7 (2008) Article DOI: 10.1016/j.bmcl.2007.10.082 BindingDB Entry DOI: 10.7270/Q2RX9CXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||