

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50346862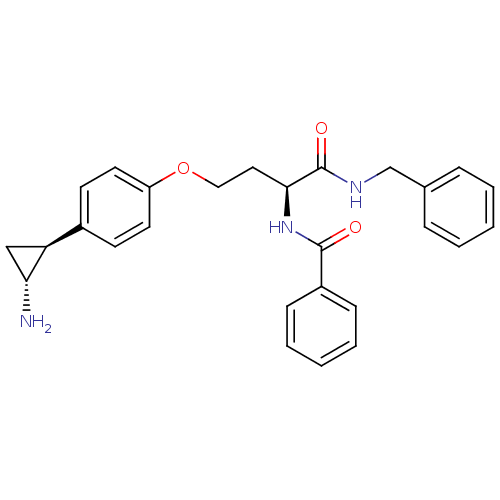 (CHEMBL1215658) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of N-terminal hexahistidine-tagged human LSD1 (1 to 852 amino acid residues) expressed in Escherichia coli BL21 (DE3) using H3K4me2 peptid... | J Med Chem 58: 7611-33 (2015) Article DOI: 10.1021/acs.jmedchem.5b00229 BindingDB Entry DOI: 10.7270/Q2QZ2CSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50346862 (CHEMBL1215658) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of N terminal hexahistidine-tag recombinant human LSD1 expressed in Escherichia coli BL21 (DE3) using histone H3 peptide as substrate prei... | Eur J Med Chem 56: 179-194 (2012) Article DOI: 10.1016/j.ejmech.2012.08.010 BindingDB Entry DOI: 10.7270/Q2TQ62NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50346862 (CHEMBL1215658) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Inhibition of LSD1 | Bioorg Med Chem 19: 3625-36 (2011) Article DOI: 10.1016/j.bmc.2011.01.046 BindingDB Entry DOI: 10.7270/Q23X870S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50346862 (CHEMBL1215658) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of human MAO-A using (4S)-4,5-dihydro-2-(6-hydroxybenzothiazolyl)-4-thiazolecarboxylic acid as substrate after 60 mins by MAO-Glo assay | J Med Chem 58: 7611-33 (2015) Article DOI: 10.1021/acs.jmedchem.5b00229 BindingDB Entry DOI: 10.7270/Q2QZ2CSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50346862 (CHEMBL1215658) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of human MAO-B using (4S)-4,5-dihydro-2-(6-hydroxybenzothiazolyl)-4-thiazolecarboxylic acid as substrate after 60 mins by MAO-Glo assay | J Med Chem 58: 7611-33 (2015) Article DOI: 10.1021/acs.jmedchem.5b00229 BindingDB Entry DOI: 10.7270/Q2QZ2CSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||