

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM50355500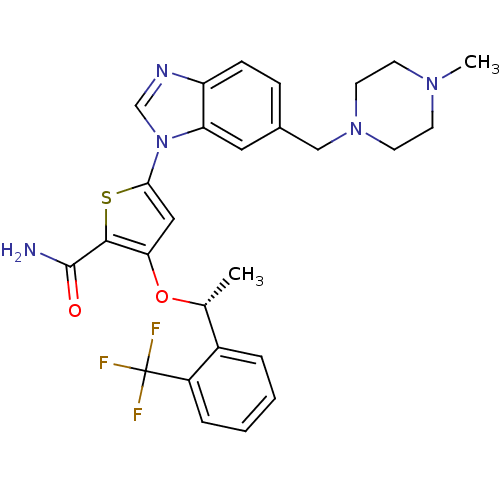 (CHEMBL1908394 | US9695172, GSK461364) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Moffitt Cancer Center Curated by ChEMBL | Assay Description Inhibition of PLK1 in HEK293T cells assessed as decrease in TCTP phosphorylation after 6 hrs by Western blot method | J Med Chem 60: 7863-7875 (2017) Article DOI: 10.1021/acs.jmedchem.7b00996 BindingDB Entry DOI: 10.7270/Q26112G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM50355500 (CHEMBL1908394 | US9695172, GSK461364) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Moffitt Cancer Center Curated by ChEMBL | Assay Description Inhibition of PLK1 in human MDA-MB-23 cells assessed as decrease in TCTP phosphorylation after 6 hrs by Western blot method | J Med Chem 60: 7863-7875 (2017) Article DOI: 10.1021/acs.jmedchem.7b00996 BindingDB Entry DOI: 10.7270/Q26112G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50355500 (CHEMBL1908394 | US9695172, GSK461364) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description Assays were performed with minor modifications from the manufacturer's protocol (PerkinElmer, USA). All reagents were diluted in 50 mM HEPES, 150 mM ... | US Patent US9695172 (2017) BindingDB Entry DOI: 10.7270/Q2JD4TX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain testis-specific protein (Homo sapiens (Human)) | BDBM50355500 (CHEMBL1908394 | US9695172, GSK461364) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description Assays were performed with minor modifications from the manufacturer's protocol (PerkinElmer, USA). All reagents were diluted in 50 mM HEPES, 150 mM ... | US Patent US9695172 (2017) BindingDB Entry DOI: 10.7270/Q2JD4TX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||