 Found 13 hits of ic50 for monomerid = 50380377
Found 13 hits of ic50 for monomerid = 50380377 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50380377
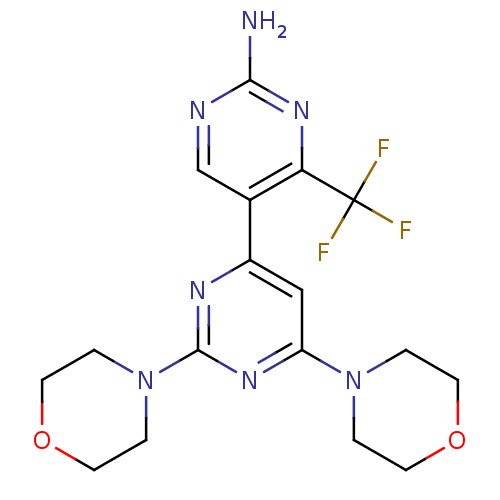
(CHEMBL2017977 | WO2007/084786, Compound 85)Show SMILES Nc1ncc(-c2cc(nc(n2)N2CCOCC2)N2CCOCC2)c(n1)C(F)(F)F Show InChI InChI=1S/C17H20F3N7O2/c18-17(19,20)14-11(10-22-15(21)25-14)12-9-13(26-1-5-28-6-2-26)24-16(23-12)27-3-7-29-8-4-27/h9-10H,1-8H2,(H2,21,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha using 1-alpha-phosphotidylinositol as substrate by ATP depletion assay |
ACS Med Chem Lett 2: 774-779 (2011)
Article DOI: 10.1021/ml200156t
BindingDB Entry DOI: 10.7270/Q2N29XXW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50380377

(CHEMBL2017977 | WO2007/084786, Compound 85)Show SMILES Nc1ncc(-c2cc(nc(n2)N2CCOCC2)N2CCOCC2)c(n1)C(F)(F)F Show InChI InChI=1S/C17H20F3N7O2/c18-17(19,20)14-11(10-22-15(21)25-14)12-9-13(26-1-5-28-6-2-26)24-16(23-12)27-3-7-29-8-4-27/h9-10H,1-8H2,(H2,21,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
The luminescence-based ATP detection reagent KinaseGlo was obtained from Promega, (Cat. No. V6714, Lot No. 236161) through Catalys, Wallisellen, Swit... |
US Patent US10202371 (2019)
BindingDB Entry DOI: 10.7270/Q2KD2123 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50380377

(CHEMBL2017977 | WO2007/084786, Compound 85)Show SMILES Nc1ncc(-c2cc(nc(n2)N2CCOCC2)N2CCOCC2)c(n1)C(F)(F)F Show InChI InChI=1S/C17H20F3N7O2/c18-17(19,20)14-11(10-22-15(21)25-14)12-9-13(26-1-5-28-6-2-26)24-16(23-12)27-3-7-29-8-4-27/h9-10H,1-8H2,(H2,21,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
The luminescence-based ATP detection reagent KinaseGlo was obtained from Promega, (Cat. No. V6714, Lot No. 236161) through Catalys, Wallisellen, Swit... |
US Patent US10202371 (2019)
BindingDB Entry DOI: 10.7270/Q2KD2123 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50380377

(CHEMBL2017977 | WO2007/084786, Compound 85)Show SMILES Nc1ncc(-c2cc(nc(n2)N2CCOCC2)N2CCOCC2)c(n1)C(F)(F)F Show InChI InChI=1S/C17H20F3N7O2/c18-17(19,20)14-11(10-22-15(21)25-14)12-9-13(26-1-5-28-6-2-26)24-16(23-12)27-3-7-29-8-4-27/h9-10H,1-8H2,(H2,21,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| |
US Patent US10202371 (2019)
BindingDB Entry DOI: 10.7270/Q2KD2123 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Rattus norvegicus (Rat)) | BDBM50380377

(CHEMBL2017977 | WO2007/084786, Compound 85)Show SMILES Nc1ncc(-c2cc(nc(n2)N2CCOCC2)N2CCOCC2)c(n1)C(F)(F)F Show InChI InChI=1S/C17H20F3N7O2/c18-17(19,20)14-11(10-22-15(21)25-14)12-9-13(26-1-5-28-6-2-26)24-16(23-12)27-3-7-29-8-4-27/h9-10H,1-8H2,(H2,21,22,25) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
AlphaScreen (Amplified Luminescent Proximity Homogeneous Assay, ALPHA, Perkin Elmer) is a non-radioactive bead-based proximity assay technology to st... |
US Patent US10202371 (2019)
BindingDB Entry DOI: 10.7270/Q2KD2123 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Rattus norvegicus (Rat)) | BDBM50380377

(CHEMBL2017977 | WO2007/084786, Compound 85)Show SMILES Nc1ncc(-c2cc(nc(n2)N2CCOCC2)N2CCOCC2)c(n1)C(F)(F)F Show InChI InChI=1S/C17H20F3N7O2/c18-17(19,20)14-11(10-22-15(21)25-14)12-9-13(26-1-5-28-6-2-26)24-16(23-12)27-3-7-29-8-4-27/h9-10H,1-8H2,(H2,21,22,25) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00267
BindingDB Entry DOI: 10.7270/Q28P64KJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Mus musculus (Mouse)) | BDBM50380377

(CHEMBL2017977 | WO2007/084786, Compound 85)Show SMILES Nc1ncc(-c2cc(nc(n2)N2CCOCC2)N2CCOCC2)c(n1)C(F)(F)F Show InChI InChI=1S/C17H20F3N7O2/c18-17(19,20)14-11(10-22-15(21)25-14)12-9-13(26-1-5-28-6-2-26)24-16(23-12)27-3-7-29-8-4-27/h9-10H,1-8H2,(H2,21,22,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 139 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
AlphaScreen (Amplified Luminescent Proximity Homogeneous Assay, ALPHA, Perkin Elmer) is a non-radioactive bead-based proximity assay technology to st... |
US Patent US10202371 (2019)
BindingDB Entry DOI: 10.7270/Q2KD2123 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Rattus norvegicus (Rat)) | BDBM50380377

(CHEMBL2017977 | WO2007/084786, Compound 85)Show SMILES Nc1ncc(-c2cc(nc(n2)N2CCOCC2)N2CCOCC2)c(n1)C(F)(F)F Show InChI InChI=1S/C17H20F3N7O2/c18-17(19,20)14-11(10-22-15(21)25-14)12-9-13(26-1-5-28-6-2-26)24-16(23-12)27-3-7-29-8-4-27/h9-10H,1-8H2,(H2,21,22,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 156 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
AlphaScreen (Amplified Luminescent Proximity Homogeneous Assay, ALPHA, Perkin Elmer) is a non-radioactive bead-based proximity assay technology to st... |
US Patent US10202371 (2019)
BindingDB Entry DOI: 10.7270/Q2KD2123 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Rattus norvegicus (Rat)) | BDBM50380377

(CHEMBL2017977 | WO2007/084786, Compound 85)Show SMILES Nc1ncc(-c2cc(nc(n2)N2CCOCC2)N2CCOCC2)c(n1)C(F)(F)F Show InChI InChI=1S/C17H20F3N7O2/c18-17(19,20)14-11(10-22-15(21)25-14)12-9-13(26-1-5-28-6-2-26)24-16(23-12)27-3-7-29-8-4-27/h9-10H,1-8H2,(H2,21,22,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 191 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00267
BindingDB Entry DOI: 10.7270/Q28P64KJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50380377

(CHEMBL2017977 | WO2007/084786, Compound 85)Show SMILES Nc1ncc(-c2cc(nc(n2)N2CCOCC2)N2CCOCC2)c(n1)C(F)(F)F Show InChI InChI=1S/C17H20F3N7O2/c18-17(19,20)14-11(10-22-15(21)25-14)12-9-13(26-1-5-28-6-2-26)24-16(23-12)27-3-7-29-8-4-27/h9-10H,1-8H2,(H2,21,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 315 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| |
US Patent US10202371 (2019)
BindingDB Entry DOI: 10.7270/Q2KD2123 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50380377

(CHEMBL2017977 | WO2007/084786, Compound 85)Show SMILES Nc1ncc(-c2cc(nc(n2)N2CCOCC2)N2CCOCC2)c(n1)C(F)(F)F Show InChI InChI=1S/C17H20F3N7O2/c18-17(19,20)14-11(10-22-15(21)25-14)12-9-13(26-1-5-28-6-2-26)24-16(23-12)27-3-7-29-8-4-27/h9-10H,1-8H2,(H2,21,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00267
BindingDB Entry DOI: 10.7270/Q28P64KJ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50380377

(CHEMBL2017977 | WO2007/084786, Compound 85)Show SMILES Nc1ncc(-c2cc(nc(n2)N2CCOCC2)N2CCOCC2)c(n1)C(F)(F)F Show InChI InChI=1S/C17H20F3N7O2/c18-17(19,20)14-11(10-22-15(21)25-14)12-9-13(26-1-5-28-6-2-26)24-16(23-12)27-3-7-29-8-4-27/h9-10H,1-8H2,(H2,21,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
TR-FRET assays for protein kinases uses a long-lifetime lanthanide Terbium or Europium chelates as the donor species which overcome interference by c... |
US Patent US10202371 (2019)
BindingDB Entry DOI: 10.7270/Q2KD2123 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50380377

(CHEMBL2017977 | WO2007/084786, Compound 85)Show SMILES Nc1ncc(-c2cc(nc(n2)N2CCOCC2)N2CCOCC2)c(n1)C(F)(F)F Show InChI InChI=1S/C17H20F3N7O2/c18-17(19,20)14-11(10-22-15(21)25-14)12-9-13(26-1-5-28-6-2-26)24-16(23-12)27-3-7-29-8-4-27/h9-10H,1-8H2,(H2,21,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00267
BindingDB Entry DOI: 10.7270/Q28P64KJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data