

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (Homo sapiens (Human)) | BDBM50398649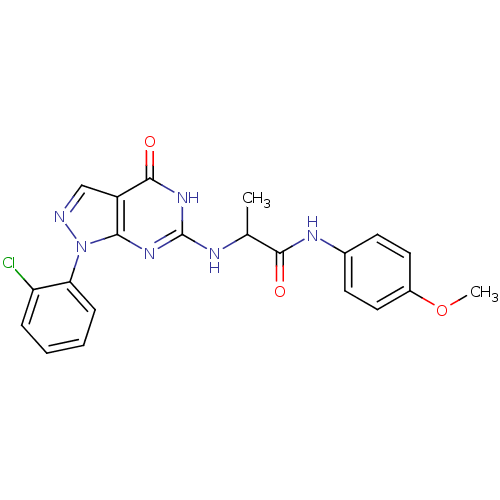 (CHEMBL2178115 | US9617269, Compound WYQ-94-D) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University; University of North Carolina at Chapel Hill US Patent | Assay Description Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera... | US Patent US9617269 (2017) BindingDB Entry DOI: 10.7270/Q2RX9F4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (Homo sapiens (Human)) | BDBM50398649 (CHEMBL2178115 | US9617269, Compound WYQ-94-D) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University Curated by ChEMBL | Assay Description Binding affinity to PDE9A2 catalytic domain (181 to 506 amino acid residues) expressed in Escherichia coli BL21 using [3H]cGMP as substrate after 15 ... | J Med Chem 55: 8549-58 (2012) Article DOI: 10.1021/jm301189c BindingDB Entry DOI: 10.7270/Q2SX6FB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50398649 (CHEMBL2178115 | US9617269, Compound WYQ-94-D) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University Curated by ChEMBL | Assay Description Binding affinity to human PDE5A1 catalytic domain (535 to 860 amino acid residues) expressed in Escherichia coli BL21 using [3H]cGMP as substrate aft... | J Med Chem 55: 8549-58 (2012) Article DOI: 10.1021/jm301189c BindingDB Entry DOI: 10.7270/Q2SX6FB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50398649 (CHEMBL2178115 | US9617269, Compound WYQ-94-D) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University Curated by ChEMBL | Assay Description Binding affinity to PDE4D2 catalytic domain (86 to 413 amino acid residues) expressed in Escherichia coli BL21 using [3H]cGMP as substrate after 15 m... | J Med Chem 55: 8549-58 (2012) Article DOI: 10.1021/jm301189c BindingDB Entry DOI: 10.7270/Q2SX6FB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1B (Homo sapiens (Human)) | BDBM50398649 (CHEMBL2178115 | US9617269, Compound WYQ-94-D) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University Curated by ChEMBL | Assay Description Binding affinity to PDE1B catalytic domain (1 to 516 amino acid residues) using [3H]cGMP as substrate after 15 mins by liquid scintillation counter | J Med Chem 55: 8549-58 (2012) Article DOI: 10.1021/jm301189c BindingDB Entry DOI: 10.7270/Q2SX6FB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A (Homo sapiens (Human)) | BDBM50398649 (CHEMBL2178115 | US9617269, Compound WYQ-94-D) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University Curated by ChEMBL | Assay Description Binding affinity to PDE3A (679 to 1087 amino acid residues) using [3H]cGMP as substrate after 15 mins by liquid scintillation counter | J Med Chem 55: 8549-58 (2012) Article DOI: 10.1021/jm301189c BindingDB Entry DOI: 10.7270/Q2SX6FB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50398649 (CHEMBL2178115 | US9617269, Compound WYQ-94-D) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University Curated by ChEMBL | Assay Description Binding affinity to PDE2A (580 to 941 amino acid residues) using [3H]cGMP as substrate after 15 mins by liquid scintillation counter | J Med Chem 55: 8549-58 (2012) Article DOI: 10.1021/jm301189c BindingDB Entry DOI: 10.7270/Q2SX6FB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50398649 (CHEMBL2178115 | US9617269, Compound WYQ-94-D) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University Curated by ChEMBL | Assay Description Binding affinity to human PDE10A2 catalytic domain (448 to 789 amino acid residues) expressed in Escherichia coli BL21 using [3H]cGMP as substrate af... | J Med Chem 55: 8549-58 (2012) Article DOI: 10.1021/jm301189c BindingDB Entry DOI: 10.7270/Q2SX6FB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A (Homo sapiens (Human)) | BDBM50398649 (CHEMBL2178115 | US9617269, Compound WYQ-94-D) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University Curated by ChEMBL | Assay Description Binding affinity to PDE8A1 catalytic domain (480 to 820 amino acid residues) using [3H]cGMP as substrate after 15 mins by liquid scintillation counte... | J Med Chem 55: 8549-58 (2012) Article DOI: 10.1021/jm301189c BindingDB Entry DOI: 10.7270/Q2SX6FB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM50398649 (CHEMBL2178115 | US9617269, Compound WYQ-94-D) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University Curated by ChEMBL | Assay Description Binding affinity to PDE7A1 catalytic domain (130 to 482 amino acid residues) using [3H]cGMP as substrate after 15 mins by liquid scintillation counte... | J Med Chem 55: 8549-58 (2012) Article DOI: 10.1021/jm301189c BindingDB Entry DOI: 10.7270/Q2SX6FB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||