

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Homo sapiens (Human)) | BDBM13280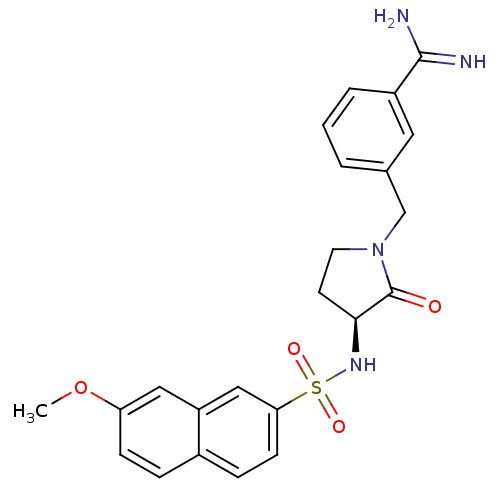 (3-{[(3S)-3-[(7-methoxynaphthalene-2-)sulfonamido]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 47 | -41.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13280 (3-{[(3S)-3-[(7-methoxynaphthalene-2-)sulfonamido]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of human Coagulation factor Xa | J Med Chem 42: 3557-71 (1999) Article DOI: 10.1021/jm990040h BindingDB Entry DOI: 10.7270/Q2M04640 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13280 (3-{[(3S)-3-[(7-methoxynaphthalene-2-)sulfonamido]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of factor 10a | J Med Chem 53: 6243-74 (2010) Article DOI: 10.1021/jm100146h BindingDB Entry DOI: 10.7270/Q2CR5VBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13280 (3-{[(3S)-3-[(7-methoxynaphthalene-2-)sulfonamido]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity against serine protease Coagulation factor X | Bioorg Med Chem Lett 9: 2753-8 (1999) BindingDB Entry DOI: 10.7270/Q2W66K02 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Bos taurus) | BDBM13280 (3-{[(3S)-3-[(7-methoxynaphthalene-2-)sulfonamido]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro evaluation of inhibitory activity against Coagulation factor X in prothrombinase complex | Bioorg Med Chem Lett 9: 2539-44 (1999) BindingDB Entry DOI: 10.7270/Q21R6R1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anionic trypsin (Bos taurus) | BDBM13280 (3-{[(3S)-3-[(7-methoxynaphthalene-2-)sulfonamido]-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description Inhibitory activity against bovine pancreatic trypsin | J Med Chem 42: 3557-71 (1999) Article DOI: 10.1021/jm990040h BindingDB Entry DOI: 10.7270/Q2M04640 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM13280 (3-{[(3S)-3-[(7-methoxynaphthalene-2-)sulfonamido]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro evaluation of inhibitory activity against trypsin | Bioorg Med Chem Lett 9: 2539-44 (1999) BindingDB Entry DOI: 10.7270/Q21R6R1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM13280 (3-{[(3S)-3-[(7-methoxynaphthalene-2-)sulfonamido]-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 853 | -34.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin K-dependent protein C (Homo sapiens (Human)) | BDBM13280 (3-{[(3S)-3-[(7-methoxynaphthalene-2-)sulfonamido]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of activated protein C (aPC) | J Med Chem 42: 3557-71 (1999) Article DOI: 10.1021/jm990040h BindingDB Entry DOI: 10.7270/Q2M04640 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM13280 (3-{[(3S)-3-[(7-methoxynaphthalene-2-)sulfonamido]-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description Inhibition of human thrombin | J Med Chem 42: 3557-71 (1999) Article DOI: 10.1021/jm990040h BindingDB Entry DOI: 10.7270/Q2M04640 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM13280 (3-{[(3S)-3-[(7-methoxynaphthalene-2-)sulfonamido]-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro evaluation of inhibitory activity against Coagulation factor II in prothrombinase complex | Bioorg Med Chem Lett 9: 2539-44 (1999) BindingDB Entry DOI: 10.7270/Q21R6R1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM13280 (3-{[(3S)-3-[(7-methoxynaphthalene-2-)sulfonamido]-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of plasmin | J Med Chem 42: 3557-71 (1999) Article DOI: 10.1021/jm990040h BindingDB Entry DOI: 10.7270/Q2M04640 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM13280 (3-{[(3S)-3-[(7-methoxynaphthalene-2-)sulfonamido]-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Tissue type plasminogen activator (tissue plasminogen activator) | J Med Chem 42: 3557-71 (1999) Article DOI: 10.1021/jm990040h BindingDB Entry DOI: 10.7270/Q2M04640 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||