

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM14723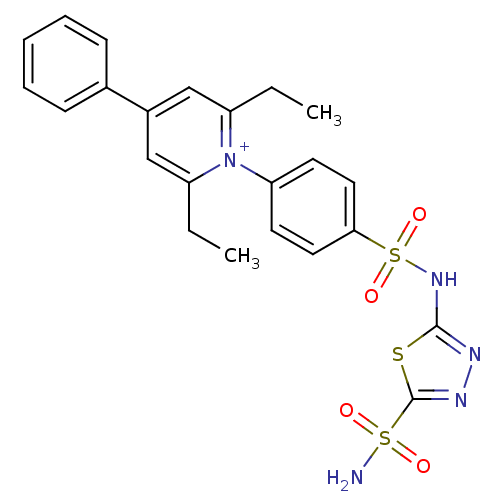 (1-N-[5-Sulfamoyl-1,3,4-thiadiazol-2-yl-(aminosulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Canadian Institutes of Health Research | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 2337-47 (2004) Article DOI: 10.1021/jm031079w BindingDB Entry DOI: 10.7270/Q2DV1H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM14723 (1-N-[5-Sulfamoyl-1,3,4-thiadiazol-2-yl-(aminosulfo...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Canadian Institutes of Health Research | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 2337-47 (2004) Article DOI: 10.1021/jm031079w BindingDB Entry DOI: 10.7270/Q2DV1H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM14723 (1-N-[5-Sulfamoyl-1,3,4-thiadiazol-2-yl-(aminosulfo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4 | -47.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Canadian Institutes of Health Research | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 2337-47 (2004) Article DOI: 10.1021/jm031079w BindingDB Entry DOI: 10.7270/Q2DV1H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM14723 (1-N-[5-Sulfamoyl-1,3,4-thiadiazol-2-yl-(aminosulfo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Canadian Institutes of Health Research | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 47: 2337-47 (2004) Article DOI: 10.1021/jm031079w BindingDB Entry DOI: 10.7270/Q2DV1H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||