 Found 3 hits of ki for monomerid = 15131
Found 3 hits of ki for monomerid = 15131 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D]
(Homo sapiens (Human)) | BDBM15131
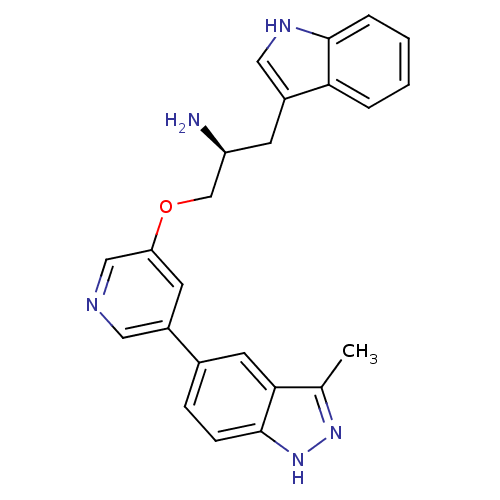
(5-indazolyl pyridine 3 | 5-{5-[(2S)-2-amino-3-(1H-...)Show SMILES Cc1n[nH]c2ccc(cc12)-c1cncc(OC[C@@H](N)Cc2c[nH]c3ccccc23)c1 |r| Show InChI InChI=1S/C24H23N5O/c1-15-22-10-16(6-7-24(22)29-28-15)17-9-20(13-26-11-17)30-14-19(25)8-18-12-27-23-5-3-2-4-21(18)23/h2-7,9-13,19,27H,8,14,25H2,1H3,(H,28,29)/t19-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.160 | -55.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories
| Assay Description
The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... |
Bioorg Med Chem Lett 16: 3740-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.046
BindingDB Entry DOI: 10.7270/Q2ZP44C9 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM15131

(5-indazolyl pyridine 3 | 5-{5-[(2S)-2-amino-3-(1H-...)Show SMILES Cc1n[nH]c2ccc(cc12)-c1cncc(OC[C@@H](N)Cc2c[nH]c3ccccc23)c1 |r| Show InChI InChI=1S/C24H23N5O/c1-15-22-10-16(6-7-24(22)29-28-15)17-9-20(13-26-11-17)30-14-19(25)8-18-12-27-23-5-3-2-4-21(18)23/h2-7,9-13,19,27H,8,14,25H2,1H3,(H,28,29)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01467
BindingDB Entry DOI: 10.7270/Q24Q801K |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM15131

(5-indazolyl pyridine 3 | 5-{5-[(2S)-2-amino-3-(1H-...)Show SMILES Cc1n[nH]c2ccc(cc12)-c1cncc(OC[C@@H](N)Cc2c[nH]c3ccccc23)c1 |r| Show InChI InChI=1S/C24H23N5O/c1-15-22-10-16(6-7-24(22)29-28-15)17-9-20(13-26-11-17)30-14-19(25)8-18-12-27-23-5-3-2-4-21(18)23/h2-7,9-13,19,27H,8,14,25H2,1H3,(H,28,29)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of AKT1 |
Nat Chem Biol 5: 484-93 (2009)
Article DOI: 10.1038/nchembio.183
BindingDB Entry DOI: 10.7270/Q2D21XTB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data