 Found 17 hits of ki for monomerid = 22888
Found 17 hits of ki for monomerid = 22888 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22888
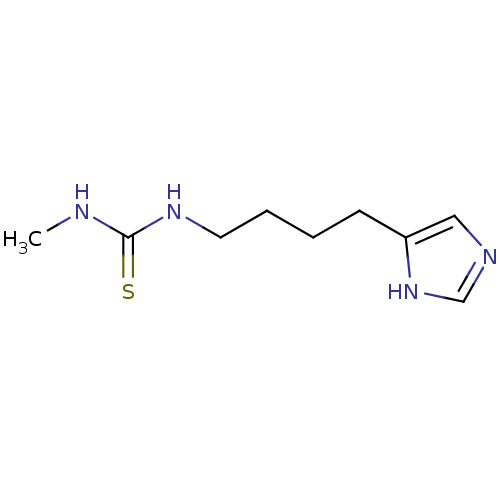
(1-[4-(1H-imidazol-5-yl)butyl]-3-methylthiourea | B...)Show InChI InChI=1S/C9H16N4S/c1-10-9(14)12-5-3-2-4-8-6-11-7-13-8/h6-7H,2-5H2,1H3,(H,11,13)(H2,10,12,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam
| Assay Description
Ligand displacement assays were performed on The SK-N-MC/hH3R cell homogenates. Retained radioactivity was determined by liquid scintillation countin... |
J Pharmacol Exp Ther 314: 1310-21 (2005)
Article DOI: 10.1124/jpet.105.087965
BindingDB Entry DOI: 10.7270/Q2KD1W6V |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM22888

(1-[4-(1H-imidazol-5-yl)butyl]-3-methylthiourea | B...)Show InChI InChI=1S/C9H16N4S/c1-10-9(14)12-5-3-2-4-8-6-11-7-13-8/h6-7H,2-5H2,1H3,(H,11,13)(H2,10,12,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 40 | -43.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Vrije Universiteit Amsterdam
| Assay Description
Ligand displacement assays were performed on The SK-N-MC/hH4R cell homogenates. Retained radioactivity was determined by liquid scintillation countin... |
J Pharmacol Exp Ther 314: 1310-21 (2005)
Article DOI: 10.1124/jpet.105.087965
BindingDB Entry DOI: 10.7270/Q2KD1W6V |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM22888

(1-[4-(1H-imidazol-5-yl)butyl]-3-methylthiourea | B...)Show InChI InChI=1S/C9H16N4S/c1-10-9(14)12-5-3-2-4-8-6-11-7-13-8/h6-7H,2-5H2,1H3,(H,11,13)(H2,10,12,14) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 47.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 299: 908-14 (2001)
BindingDB Entry DOI: 10.7270/Q2JS9P1N |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM22888

(1-[4-(1H-imidazol-5-yl)butyl]-3-methylthiourea | B...)Show InChI InChI=1S/C9H16N4S/c1-10-9(14)12-5-3-2-4-8-6-11-7-13-8/h6-7H,2-5H2,1H3,(H,11,13)(H2,10,12,14) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 63.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research & Development Ltd.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 311: 305-10 (1996)
Article DOI: 10.1016/0014-2999(96)00428-1
BindingDB Entry DOI: 10.7270/Q2ZG6QSD |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM22888

(1-[4-(1H-imidazol-5-yl)butyl]-3-methylthiourea | B...)Show InChI InChI=1S/C9H16N4S/c1-10-9(14)12-5-3-2-4-8-6-11-7-13-8/h6-7H,2-5H2,1H3,(H,11,13)(H2,10,12,14) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
U. 109
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 271: 452-9 (1994)
BindingDB Entry DOI: 10.7270/Q23X8556 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM22888

(1-[4-(1H-imidazol-5-yl)butyl]-3-methylthiourea | B...)Show InChI InChI=1S/C9H16N4S/c1-10-9(14)12-5-3-2-4-8-6-11-7-13-8/h6-7H,2-5H2,1H3,(H,11,13)(H2,10,12,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
In vitro antagonism of the histamine H3-receptor in the rat cerebral cortex. |
J Med Chem 38: 3342-50 (1995)
BindingDB Entry DOI: 10.7270/Q2CN72X5 |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM22888

(1-[4-(1H-imidazol-5-yl)butyl]-3-methylthiourea | B...)Show InChI InChI=1S/C9H16N4S/c1-10-9(14)12-5-3-2-4-8-6-11-7-13-8/h6-7H,2-5H2,1H3,(H,11,13)(H2,10,12,14) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
U. 109
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 271: 452-9 (1994)
BindingDB Entry DOI: 10.7270/Q23X8556 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22888

(1-[4-(1H-imidazol-5-yl)butyl]-3-methylthiourea | B...)Show InChI InChI=1S/C9H16N4S/c1-10-9(14)12-5-3-2-4-8-6-11-7-13-8/h6-7H,2-5H2,1H3,(H,11,13)(H2,10,12,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 77.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 299: 908-14 (2001)
BindingDB Entry DOI: 10.7270/Q2JS9P1N |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22888

(1-[4-(1H-imidazol-5-yl)butyl]-3-methylthiourea | B...)Show InChI InChI=1S/C9H16N4S/c1-10-9(14)12-5-3-2-4-8-6-11-7-13-8/h6-7H,2-5H2,1H3,(H,11,13)(H2,10,12,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to the human histamine H3 receptor |
J Med Chem 46: 3957-60 (2003)
Article DOI: 10.1021/jm0341047
BindingDB Entry DOI: 10.7270/Q2QJ7J1C |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22888

(1-[4-(1H-imidazol-5-yl)butyl]-3-methylthiourea | B...)Show InChI InChI=1S/C9H16N4S/c1-10-9(14)12-5-3-2-4-8-6-11-7-13-8/h6-7H,2-5H2,1H3,(H,11,13)(H2,10,12,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute
Curated by PDSP Ki Database
| |
Mol Pharmacol 59: 420-6 (2001)
Article DOI: 10.1124/mol.59.3.420
BindingDB Entry DOI: 10.7270/Q26Q1VSR |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM22888

(1-[4-(1H-imidazol-5-yl)butyl]-3-methylthiourea | B...)Show InChI InChI=1S/C9H16N4S/c1-10-9(14)12-5-3-2-4-8-6-11-7-13-8/h6-7H,2-5H2,1H3,(H,11,13)(H2,10,12,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to the human histamine H4 receptor |
J Med Chem 46: 3957-60 (2003)
Article DOI: 10.1021/jm0341047
BindingDB Entry DOI: 10.7270/Q2QJ7J1C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM22888

(1-[4-(1H-imidazol-5-yl)butyl]-3-methylthiourea | B...)Show InChI InChI=1S/C9H16N4S/c1-10-9(14)12-5-3-2-4-8-6-11-7-13-8/h6-7H,2-5H2,1H3,(H,11,13)(H2,10,12,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute
Curated by PDSP Ki Database
| |
Mol Pharmacol 59: 420-6 (2001)
Article DOI: 10.1124/mol.59.3.420
BindingDB Entry DOI: 10.7270/Q26Q1VSR |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM22888

(1-[4-(1H-imidazol-5-yl)butyl]-3-methylthiourea | B...)Show InChI InChI=1S/C9H16N4S/c1-10-9(14)12-5-3-2-4-8-6-11-7-13-8/h6-7H,2-5H2,1H3,(H,11,13)(H2,10,12,14) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
U. 109
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 271: 452-9 (1994)
BindingDB Entry DOI: 10.7270/Q23X8556 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM22888

(1-[4-(1H-imidazol-5-yl)butyl]-3-methylthiourea | B...)Show InChI InChI=1S/C9H16N4S/c1-10-9(14)12-5-3-2-4-8-6-11-7-13-8/h6-7H,2-5H2,1H3,(H,11,13)(H2,10,12,14) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by PDSP Ki Database
| |
Mol Pharmacol 13: 454-73 (1977)
BindingDB Entry DOI: 10.7270/Q2TT4PFZ |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM22888

(1-[4-(1H-imidazol-5-yl)butyl]-3-methylthiourea | B...)Show InChI InChI=1S/C9H16N4S/c1-10-9(14)12-5-3-2-4-8-6-11-7-13-8/h6-7H,2-5H2,1H3,(H,11,13)(H2,10,12,14) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by PDSP Ki Database
| |
Mol Pharmacol 13: 454-73 (1977)
BindingDB Entry DOI: 10.7270/Q2TT4PFZ |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Cavia porcellus (domestic guinea pig)) | BDBM22888

(1-[4-(1H-imidazol-5-yl)butyl]-3-methylthiourea | B...)Show InChI InChI=1S/C9H16N4S/c1-10-9(14)12-5-3-2-4-8-6-11-7-13-8/h6-7H,2-5H2,1H3,(H,11,13)(H2,10,12,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Pharmaceutical Research Laboratories
Curated by PDSP Ki Database
| |
Nature 304: 65-7 (1983)
Article DOI: 10.1038/304065a0
BindingDB Entry DOI: 10.7270/Q2930RN2 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM22888

(1-[4-(1H-imidazol-5-yl)butyl]-3-methylthiourea | B...)Show InChI InChI=1S/C9H16N4S/c1-10-9(14)12-5-3-2-4-8-6-11-7-13-8/h6-7H,2-5H2,1H3,(H,11,13)(H2,10,12,14) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 75: 6290-4 (1978)
Article DOI: 10.1073/pnas.75.12.6290
BindingDB Entry DOI: 10.7270/Q2FN14P6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data