

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23887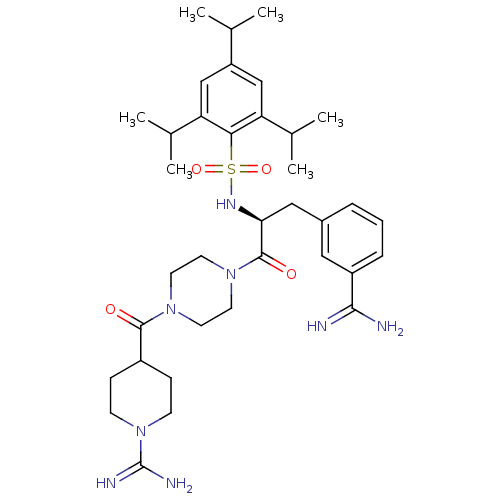 (3-amidinophenylalanine deriv., 31 | 4-({4-[(2S)-3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of matripase (unknown origin) using Boc-QAR-AMC as substrate incubated for 30 mins prior to substrate addition by fluorescence assay | Bioorg Med Chem 23: 2328-43 (2015) Article DOI: 10.1016/j.bmc.2015.03.072 BindingDB Entry DOI: 10.7270/Q23F4RB4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23887 (3-amidinophenylalanine deriv., 31 | 4-({4-[(2S)-3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 14 | -44.8 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM23887 (3-amidinophenylalanine deriv., 31 | 4-({4-[(2S)-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM23887 (3-amidinophenylalanine deriv., 31 | 4-({4-[(2S)-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM23887 (3-amidinophenylalanine deriv., 31 | 4-({4-[(2S)-3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of hepsin (unknown origin) using Boc-QAR-AMC as substrate after 30 mins prior to substrate addition by fluorescence assay | Bioorg Med Chem 23: 2328-43 (2015) Article DOI: 10.1016/j.bmc.2015.03.072 BindingDB Entry DOI: 10.7270/Q23F4RB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM23887 (3-amidinophenylalanine deriv., 31 | 4-({4-[(2S)-3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Bos taurus) | BDBM23887 (3-amidinophenylalanine deriv., 31 | 4-({4-[(2S)-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM23887 (3-amidinophenylalanine deriv., 31 | 4-({4-[(2S)-3-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal His-tagged HGFA (unknown origin) expressed in baculovirus-infected Sf9 cells using Boc-QLR-AMC as substrate incu... | Bioorg Med Chem 23: 2328-43 (2015) Article DOI: 10.1016/j.bmc.2015.03.072 BindingDB Entry DOI: 10.7270/Q23F4RB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||