 Found 18 hits of ki for monomerid = 33280
Found 18 hits of ki for monomerid = 33280 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM33280
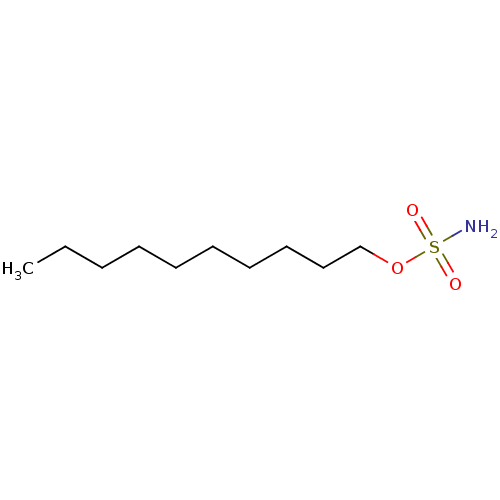
(CHEMBL153094 | aliphatic sulfamate, 2)Show InChI InChI=1S/C10H23NO3S/c1-2-3-4-5-6-7-8-9-10-14-15(11,12)13/h2-10H2,1H3,(H2,11,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Montpellier II
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II. |
J Med Chem 46: 5471-7 (2003)
Article DOI: 10.1021/jm030911u
BindingDB Entry DOI: 10.7270/Q2KP81KV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM33280

(CHEMBL153094 | aliphatic sulfamate, 2)Show InChI InChI=1S/C10H23NO3S/c1-2-3-4-5-6-7-8-9-10-14-15(11,12)13/h2-10H2,1H3,(H2,11,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.700 | -51.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 23 |
CNR
| Assay Description
An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... |
J Med Chem 52: 5990-8 (2009)
Article DOI: 10.1021/jm900641r
BindingDB Entry DOI: 10.7270/Q2GF0RVR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM33280

(CHEMBL153094 | aliphatic sulfamate, 2)Show InChI InChI=1S/C10H23NO3S/c1-2-3-4-5-6-7-8-9-10-14-15(11,12)13/h2-10H2,1H3,(H2,11,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNR
| Assay Description
An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... |
J Med Chem 52: 5990-8 (2009)
Article DOI: 10.1021/jm900641r
BindingDB Entry DOI: 10.7270/Q2GF0RVR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM33280

(CHEMBL153094 | aliphatic sulfamate, 2)Show InChI InChI=1S/C10H23NO3S/c1-2-3-4-5-6-7-8-9-10-14-15(11,12)13/h2-10H2,1H3,(H2,11,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Montpellier II
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase IX. |
J Med Chem 46: 5471-7 (2003)
Article DOI: 10.1021/jm030911u
BindingDB Entry DOI: 10.7270/Q2KP81KV |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM33280

(CHEMBL153094 | aliphatic sulfamate, 2)Show InChI InChI=1S/C10H23NO3S/c1-2-3-4-5-6-7-8-9-10-14-15(11,12)13/h2-10H2,1H3,(H2,11,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 23.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNR
| Assay Description
An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... |
J Med Chem 52: 5990-8 (2009)
Article DOI: 10.1021/jm900641r
BindingDB Entry DOI: 10.7270/Q2GF0RVR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM33280

(CHEMBL153094 | aliphatic sulfamate, 2)Show InChI InChI=1S/C10H23NO3S/c1-2-3-4-5-6-7-8-9-10-14-15(11,12)13/h2-10H2,1H3,(H2,11,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 67.6 | -40.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 23 |
CNR
| Assay Description
An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... |
J Med Chem 52: 5990-8 (2009)
Article DOI: 10.1021/jm900641r
BindingDB Entry DOI: 10.7270/Q2GF0RVR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM33280

(CHEMBL153094 | aliphatic sulfamate, 2)Show InChI InChI=1S/C10H23NO3S/c1-2-3-4-5-6-7-8-9-10-14-15(11,12)13/h2-10H2,1H3,(H2,11,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 88.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNR
| Assay Description
An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... |
J Med Chem 52: 5990-8 (2009)
Article DOI: 10.1021/jm900641r
BindingDB Entry DOI: 10.7270/Q2GF0RVR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM33280

(CHEMBL153094 | aliphatic sulfamate, 2)Show InChI InChI=1S/C10H23NO3S/c1-2-3-4-5-6-7-8-9-10-14-15(11,12)13/h2-10H2,1H3,(H2,11,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 142 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Montpellier II
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase IX. |
J Med Chem 46: 5471-7 (2003)
Article DOI: 10.1021/jm030911u
BindingDB Entry DOI: 10.7270/Q2KP81KV |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM33280

(CHEMBL153094 | aliphatic sulfamate, 2)Show InChI InChI=1S/C10H23NO3S/c1-2-3-4-5-6-7-8-9-10-14-15(11,12)13/h2-10H2,1H3,(H2,11,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Montpellier II
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase I. |
J Med Chem 46: 5471-7 (2003)
Article DOI: 10.1021/jm030911u
BindingDB Entry DOI: 10.7270/Q2KP81KV |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM33280

(CHEMBL153094 | aliphatic sulfamate, 2)Show InChI InChI=1S/C10H23NO3S/c1-2-3-4-5-6-7-8-9-10-14-15(11,12)13/h2-10H2,1H3,(H2,11,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 530 | -35.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 23 |
CNR
| Assay Description
An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... |
J Med Chem 52: 5990-8 (2009)
Article DOI: 10.1021/jm900641r
BindingDB Entry DOI: 10.7270/Q2GF0RVR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 14
(Homo sapiens (Human)) | BDBM33280

(CHEMBL153094 | aliphatic sulfamate, 2)Show InChI InChI=1S/C10H23NO3S/c1-2-3-4-5-6-7-8-9-10-14-15(11,12)13/h2-10H2,1H3,(H2,11,12,13) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 711 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNR
| Assay Description
An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... |
J Med Chem 52: 5990-8 (2009)
Article DOI: 10.1021/jm900641r
BindingDB Entry DOI: 10.7270/Q2GF0RVR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 5A, mitochondrial
(Homo sapiens (Human)) | BDBM33280

(CHEMBL153094 | aliphatic sulfamate, 2)Show InChI InChI=1S/C10H23NO3S/c1-2-3-4-5-6-7-8-9-10-14-15(11,12)13/h2-10H2,1H3,(H2,11,12,13) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 805 | -34.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 23 |
CNR
| Assay Description
An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... |
J Med Chem 52: 5990-8 (2009)
Article DOI: 10.1021/jm900641r
BindingDB Entry DOI: 10.7270/Q2GF0RVR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 3
(Homo sapiens (Human)) | BDBM33280

(CHEMBL153094 | aliphatic sulfamate, 2)Show InChI InChI=1S/C10H23NO3S/c1-2-3-4-5-6-7-8-9-10-14-15(11,12)13/h2-10H2,1H3,(H2,11,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 877 | -34.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 23 |
CNR
| Assay Description
An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... |
J Med Chem 52: 5990-8 (2009)
Article DOI: 10.1021/jm900641r
BindingDB Entry DOI: 10.7270/Q2GF0RVR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 5B, mitochondrial
(Homo sapiens (Human)) | BDBM33280

(CHEMBL153094 | aliphatic sulfamate, 2)Show InChI InChI=1S/C10H23NO3S/c1-2-3-4-5-6-7-8-9-10-14-15(11,12)13/h2-10H2,1H3,(H2,11,12,13) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 888 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNR
| Assay Description
An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... |
J Med Chem 52: 5990-8 (2009)
Article DOI: 10.1021/jm900641r
BindingDB Entry DOI: 10.7270/Q2GF0RVR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 13
(Mus musculus (mouse)) | BDBM33280

(CHEMBL153094 | aliphatic sulfamate, 2)Show InChI InChI=1S/C10H23NO3S/c1-2-3-4-5-6-7-8-9-10-14-15(11,12)13/h2-10H2,1H3,(H2,11,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 956 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNR
| Assay Description
An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... |
J Med Chem 52: 5990-8 (2009)
Article DOI: 10.1021/jm900641r
BindingDB Entry DOI: 10.7270/Q2GF0RVR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 6
(Homo sapiens (Human)) | BDBM33280

(CHEMBL153094 | aliphatic sulfamate, 2)Show InChI InChI=1S/C10H23NO3S/c1-2-3-4-5-6-7-8-9-10-14-15(11,12)13/h2-10H2,1H3,(H2,11,12,13) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNR
| Assay Description
An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... |
J Med Chem 52: 5990-8 (2009)
Article DOI: 10.1021/jm900641r
BindingDB Entry DOI: 10.7270/Q2GF0RVR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM33280

(CHEMBL153094 | aliphatic sulfamate, 2)Show InChI InChI=1S/C10H23NO3S/c1-2-3-4-5-6-7-8-9-10-14-15(11,12)13/h2-10H2,1H3,(H2,11,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Montpellier II
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II. |
J Med Chem 46: 5471-7 (2003)
Article DOI: 10.1021/jm030911u
BindingDB Entry DOI: 10.7270/Q2KP81KV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM33280

(CHEMBL153094 | aliphatic sulfamate, 2)Show InChI InChI=1S/C10H23NO3S/c1-2-3-4-5-6-7-8-9-10-14-15(11,12)13/h2-10H2,1H3,(H2,11,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Montpellier II
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase I. |
J Med Chem 46: 5471-7 (2003)
Article DOI: 10.1021/jm030911u
BindingDB Entry DOI: 10.7270/Q2KP81KV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data