

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50034683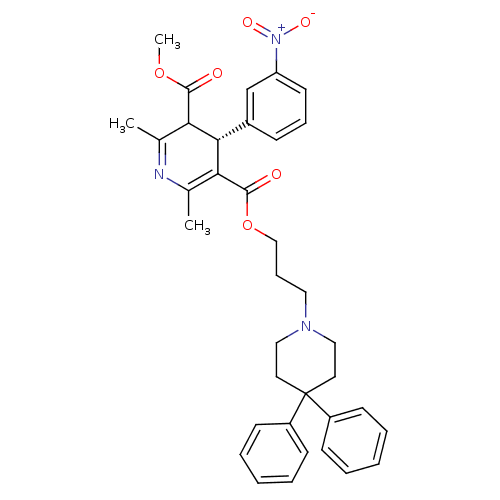 ((S)-2,6-Dimethyl-4-(3-nitro-phenyl)-1,4-dihydro-py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description Ability to displace [3H]prazosin from cloned human Alpha-1A adrenergic receptor | J Med Chem 38: 1579-81 (1995) BindingDB Entry DOI: 10.7270/Q25Q4WRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50034683 ((S)-2,6-Dimethyl-4-(3-nitro-phenyl)-1,4-dihydro-py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Binding constant measured against Alpha-1A adrenergic receptor in human prostate; +++:highly active | Bioorg Med Chem Lett 15: 657-64 (2005) Article DOI: 10.1016/j.bmcl.2004.11.032 BindingDB Entry DOI: 10.7270/Q2SN08GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1B adrenergic receptor (Homo sapiens (Human)) | BDBM50034683 ((S)-2,6-Dimethyl-4-(3-nitro-phenyl)-1,4-dihydro-py...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description Ability to displace [3H]prazosin from cloned human Alpha-1B adrenergic receptor | J Med Chem 38: 1579-81 (1995) BindingDB Entry DOI: 10.7270/Q25Q4WRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM50034683 ((S)-2,6-Dimethyl-4-(3-nitro-phenyl)-1,4-dihydro-py...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description Ability to displace [3H]prazosin from cloned human Alpha-1D adrenergic receptor | J Med Chem 38: 1579-81 (1995) BindingDB Entry DOI: 10.7270/Q25Q4WRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50034683 ((S)-2,6-Dimethyl-4-(3-nitro-phenyl)-1,4-dihydro-py...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description Ability to displace [3H]rauwolscine from cloned human Alpha-2C adrenergic receptor | J Med Chem 38: 1579-81 (1995) BindingDB Entry DOI: 10.7270/Q25Q4WRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2B adrenergic receptor (Homo sapiens (Human)) | BDBM50034683 ((S)-2,6-Dimethyl-4-(3-nitro-phenyl)-1,4-dihydro-py...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description Ability to displace [3H]rauwolscine from cloned human Alpha-2B adrenergic receptor | J Med Chem 38: 1579-81 (1995) BindingDB Entry DOI: 10.7270/Q25Q4WRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50034683 ((S)-2,6-Dimethyl-4-(3-nitro-phenyl)-1,4-dihydro-py...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description Ability to displace [3H]rauwolscine from cloned human Alpha-2A adrenergic receptor | J Med Chem 38: 1579-81 (1995) BindingDB Entry DOI: 10.7270/Q25Q4WRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50034683 ((S)-2,6-Dimethyl-4-(3-nitro-phenyl)-1,4-dihydro-py...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Binding affinity against cloned human adenosine A3 receptor by radioligand binding assay using [125I]-AB-MECA. | J Med Chem 39: 4667-75 (1996) Article DOI: 10.1021/jm960457c BindingDB Entry DOI: 10.7270/Q2VQ33BG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50034683 ((S)-2,6-Dimethyl-4-(3-nitro-phenyl)-1,4-dihydro-py...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Displacement of [125 I]AB-MECA from human Adenosine A3 receptor expressed in HEK cells | J Med Chem 42: 3055-65 (1999) Article DOI: 10.1021/jm980688e BindingDB Entry DOI: 10.7270/Q27W6BC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50034683 ((S)-2,6-Dimethyl-4-(3-nitro-phenyl)-1,4-dihydro-py...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Displacement of [125]AB-MECA binding to human Adenosine A3 receptor expressed in HEK cells | J Med Chem 39: 2980-9 (1996) Article DOI: 10.1021/jm9600205 BindingDB Entry DOI: 10.7270/Q2833SQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50034683 ((S)-2,6-Dimethyl-4-(3-nitro-phenyl)-1,4-dihydro-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Displacement of [3H]-CGS-21,680 from Adenosine A2A receptor of rat striatal membrane at 10e-4 microM | J Med Chem 42: 3055-65 (1999) Article DOI: 10.1021/jm980688e BindingDB Entry DOI: 10.7270/Q27W6BC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50034683 ((S)-2,6-Dimethyl-4-(3-nitro-phenyl)-1,4-dihydro-py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor in rat cerebral cortex membrane by radioligand binding assay using [3H](R)-PIA. | J Med Chem 39: 4667-75 (1996) Article DOI: 10.1021/jm960457c BindingDB Entry DOI: 10.7270/Q2VQ33BG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50034683 ((S)-2,6-Dimethyl-4-(3-nitro-phenyl)-1,4-dihydro-py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Displacement of [3H]-R-PIA from Adenosine A1 receptor of rat brain membrane | J Med Chem 42: 3055-65 (1999) Article DOI: 10.1021/jm980688e BindingDB Entry DOI: 10.7270/Q27W6BC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||