 Found 5 hits of ki for monomerid = 50090676
Found 5 hits of ki for monomerid = 50090676 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50090676
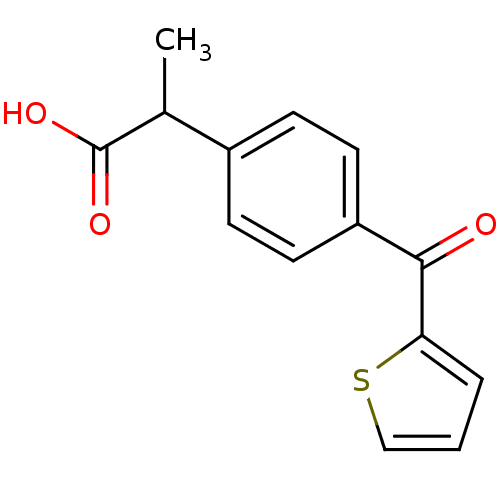
((+-)-2-(p-(2-thenoyl)phenyl)propionic acid | 2-(4-...)Show InChI InChI=1S/C14H12O3S/c1-9(14(16)17)10-4-6-11(7-5-10)13(15)12-3-2-8-18-12/h2-9H,1H3,(H,16,17) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Bartholomew's and the Royal London School of Medicine and Dentistry
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 96: 7563-8 (1999)
Article DOI: 10.1073/pnas.96.13.7563
BindingDB Entry DOI: 10.7270/Q21G0JT4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50090676

((+-)-2-(p-(2-thenoyl)phenyl)propionic acid | 2-(4-...)Show InChI InChI=1S/C14H12O3S/c1-9(14(16)17)10-4-6-11(7-5-10)13(15)12-3-2-8-18-12/h2-9H,1H3,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
| DrugBank
PubMed
| 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Mechanism based inhibition of human cytochrome P450 2C9 |
Curr Drug Metab 6: 413-54 (2005)
BindingDB Entry DOI: 10.7270/Q2VQ33X3 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50090676

((+-)-2-(p-(2-thenoyl)phenyl)propionic acid | 2-(4-...)Show InChI InChI=1S/C14H12O3S/c1-9(14(16)17)10-4-6-11(7-5-10)13(15)12-3-2-8-18-12/h2-9H,1H3,(H,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
| DrugBank
Article
PubMed
| 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Bartholomew's and the Royal London School of Medicine and Dentistry
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 96: 7563-8 (1999)
Article DOI: 10.1073/pnas.96.13.7563
BindingDB Entry DOI: 10.7270/Q21G0JT4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50090676

((+-)-2-(p-(2-thenoyl)phenyl)propionic acid | 2-(4-...)Show InChI InChI=1S/C14H12O3S/c1-9(14(16)17)10-4-6-11(7-5-10)13(15)12-3-2-8-18-12/h2-9H,1H3,(H,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
| DrugBank
Article
PubMed
| 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Bartholomew's and the Royal London School of Medicine and Dentistry
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 96: 7563-8 (1999)
Article DOI: 10.1073/pnas.96.13.7563
BindingDB Entry DOI: 10.7270/Q21G0JT4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50090676

((+-)-2-(p-(2-thenoyl)phenyl)propionic acid | 2-(4-...)Show InChI InChI=1S/C14H12O3S/c1-9(14(16)17)10-4-6-11(7-5-10)13(15)12-3-2-8-18-12/h2-9H,1H3,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
| DrugBank
PubMed
| 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by ChEMBL
| Assay Description
Binding affinity measured on human cytochrome P450 2C9 (CYP2C9) enzyme |
J Med Chem 43: 2789-96 (2000)
BindingDB Entry DOI: 10.7270/Q2K64H9Q |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data