

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50097613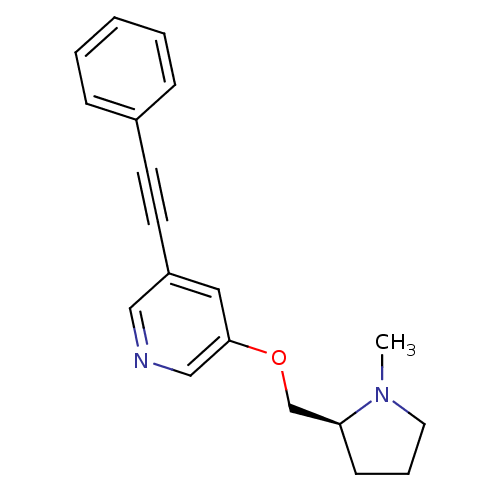 (3-((S)-1-Methyl-pyrrolidin-2-ylmethoxy)-5-phenylet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro ability to displace [3H]-(-)-cytisine binding to whole rat brain membranes at Nicotinic acetylcholine receptor alpha4-beta2 | Bioorg Med Chem Lett 11: 631-3 (2001) BindingDB Entry DOI: 10.7270/Q2WM1CPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50097613 (3-((S)-1-Methyl-pyrrolidin-2-ylmethoxy)-5-phenylet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of [3H]epibatidine binding to rat Nicotinic acetylcholine receptor alpha4-beta2 | J Med Chem 48: 1721-4 (2005) Article DOI: 10.1021/jm0492406 BindingDB Entry DOI: 10.7270/Q2BZ65JW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-2 (Homo sapiens (Human)) | BDBM50097613 (3-((S)-1-Methyl-pyrrolidin-2-ylmethoxy)-5-phenylet...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of [3H]epibatidine binding to Nicotinic acetylcholine receptor alpha3-beta2 | J Med Chem 48: 1721-4 (2005) Article DOI: 10.1021/jm0492406 BindingDB Entry DOI: 10.7270/Q2BZ65JW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Rattus norvegicus (Rat)) | BDBM50097613 (3-((S)-1-Methyl-pyrrolidin-2-ylmethoxy)-5-phenylet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of [3H]epibatidine binding to rat Nicotinic acetylcholine receptor alpha3-beta4 | J Med Chem 48: 1721-4 (2005) Article DOI: 10.1021/jm0492406 BindingDB Entry DOI: 10.7270/Q2BZ65JW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||