

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chymase (Homo sapiens (Human)) | BDBM50098853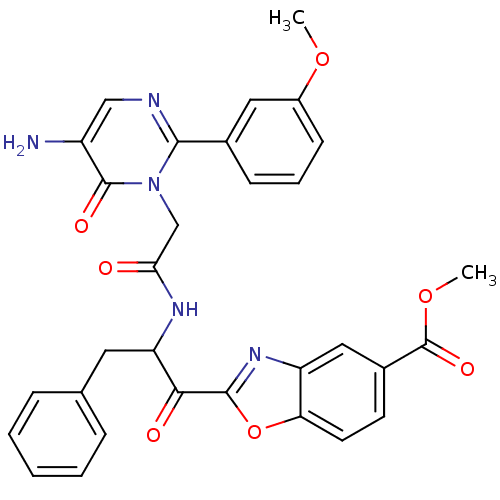 (2-(2-{2-[5-Amino-2-(3-methoxy-phenyl)-6-oxo-6H-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description Inhibitory activity against canine skin chymase | J Med Chem 44: 1286-96 (2001) BindingDB Entry DOI: 10.7270/Q26H4J4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50098853 (2-(2-{2-[5-Amino-2-(3-methoxy-phenyl)-6-oxo-6H-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description Inhibitory activity evaluated against chymase from human heart. | J Med Chem 44: 1286-96 (2001) BindingDB Entry DOI: 10.7270/Q26H4J4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast cell protease 9 (Mus musculus) | BDBM50098853 (2-(2-{2-[5-Amino-2-(3-methoxy-phenyl)-6-oxo-6H-pyr...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 63.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description Inhibitory activity against mouse peritoneal chymase | J Med Chem 44: 1286-96 (2001) BindingDB Entry DOI: 10.7270/Q26H4J4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Rattus norvegicus) | BDBM50098853 (2-(2-{2-[5-Amino-2-(3-methoxy-phenyl)-6-oxo-6H-pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 86.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description Inhibitory activity against rat peritoneal chymase | J Med Chem 44: 1286-96 (2001) BindingDB Entry DOI: 10.7270/Q26H4J4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin G (Homo sapiens (Human)) | BDBM50098853 (2-(2-{2-[5-Amino-2-(3-methoxy-phenyl)-6-oxo-6H-pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 379 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description Inhibitory activity against human cathepsin G | J Med Chem 44: 1286-96 (2001) BindingDB Entry DOI: 10.7270/Q26H4J4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50098853 (2-(2-{2-[5-Amino-2-(3-methoxy-phenyl)-6-oxo-6H-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 943 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description Inhibitory activity against alpha chymotrypsin from bovine pancreas. | J Med Chem 44: 1286-96 (2001) BindingDB Entry DOI: 10.7270/Q26H4J4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||