

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50110730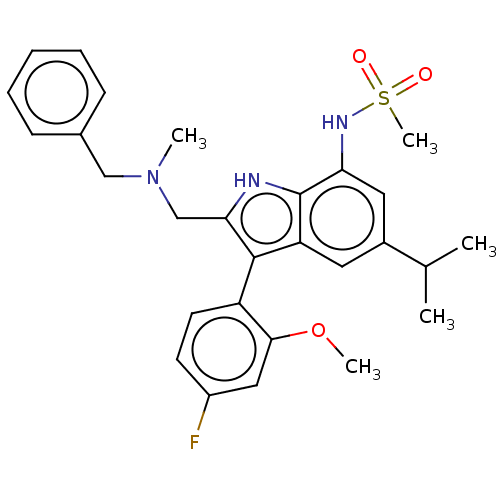 (CHEMBL3605919) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-dexamethasone from human glucocorticoid receptor expressed in HEK293 cells by scintillation counting based radioligand competiti... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50110730 (CHEMBL3605919) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-progesterone from human progesterone receptor expressed in HEK293 cells by scintillation counting based radioligand competition ... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50110730 (CHEMBL3605919) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 232 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-methyltrienolone from human androgen receptor expressed in HEK293 cells by scintillation counting based radioligand competition ... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50110730 (CHEMBL3605919) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-aldosterone from human mineralocorticoid receptor expressed in HEK293 cells by scintillation counting based radioligand competit... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||