

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122706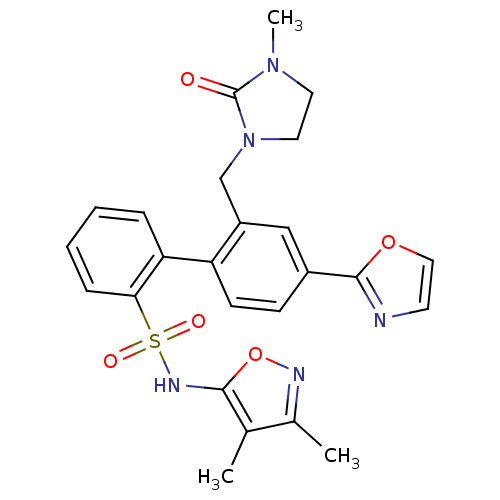 (2'-(3-Methyl-2-oxo-imidazolidin-1-ylmethyl)-4'-oxa...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Homo sapiens (Human)) | BDBM50122706 (2'-(3-Methyl-2-oxo-imidazolidin-1-ylmethyl)-4'-oxa...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin B receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||