

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50133003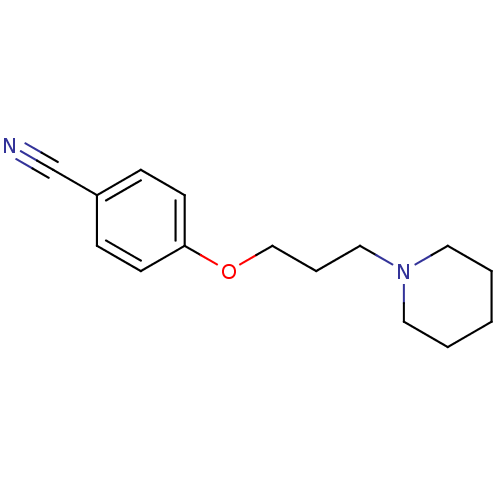 (4-(3-(piperidin-1-yl)propoxy)benzonitrile | 4-(3-P...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from human histamine H3 receptor expressed in HEL293 cells | Bioorg Med Chem Lett 19: 2172-5 (2009) Article DOI: 10.1016/j.bmcl.2009.02.110 BindingDB Entry DOI: 10.7270/Q2H41RBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50133003 (4-(3-(piperidin-1-yl)propoxy)benzonitrile | 4-(3-P...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity to the human histamine H3 receptor | J Med Chem 46: 3957-60 (2003) Article DOI: 10.1021/jm0341047 BindingDB Entry DOI: 10.7270/Q2QJ7J1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50133003 (4-(3-(piperidin-1-yl)propoxy)benzonitrile | 4-(3-P...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 25.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Mean binding affinity for human H3 receptor | J Med Chem 48: 2229-38 (2005) Article DOI: 10.1021/jm049212n BindingDB Entry DOI: 10.7270/Q2GB258T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50133003 (4-(3-(piperidin-1-yl)propoxy)benzonitrile | 4-(3-P...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by PDSP Ki Database | Mol Pharmacol 59: 420-6 (2001) Article DOI: 10.1124/mol.59.3.420 BindingDB Entry DOI: 10.7270/Q26Q1VSR | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50133003 (4-(3-(piperidin-1-yl)propoxy)benzonitrile | 4-(3-P...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity to the human histamine H4 receptor | J Med Chem 46: 3957-60 (2003) Article DOI: 10.1021/jm0341047 BindingDB Entry DOI: 10.7270/Q2QJ7J1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||