

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trypsin (Homo sapiens (Human)) | BDBM50158155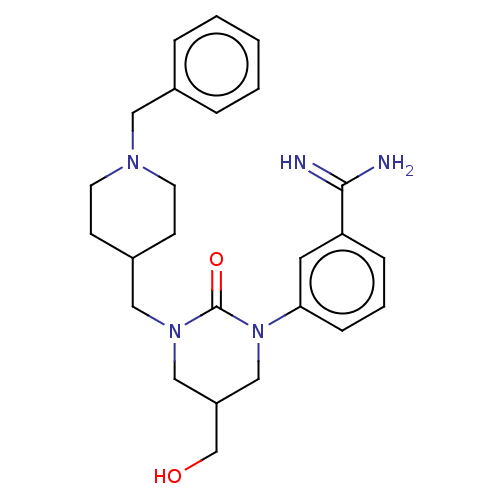 (CHEMBL3781354) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Curated by ChEMBL | Assay Description Inhibition of human recombinant trypsin using H2N(EEdansyl)GKQLRVVNGG (KDabcyl)-NH2 as substrate by fluorometric analysis | ACS Med Chem Lett 7: 177-81 (2016) Article DOI: 10.1021/acsmedchemlett.5b00357 BindingDB Entry DOI: 10.7270/Q2Z60QZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM50158155 (CHEMBL3781354) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Curated by ChEMBL | Assay Description Inhibition of human recombinant hepatocyte growth factor activator using H2N(EEdansyl)GKQLRVVNGG (KDabcyl)-NH2 as substrate by fluorometric analysis | ACS Med Chem Lett 7: 177-81 (2016) Article DOI: 10.1021/acsmedchemlett.5b00357 BindingDB Entry DOI: 10.7270/Q2Z60QZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50158155 (CHEMBL3781354) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Curated by ChEMBL | Assay Description Inhibition of human recombinant factor 10a using H2N(EEdansyl)GKQLRVVNGG (KDabcyl)-NH2 as substrate by fluorometric analysis | ACS Med Chem Lett 7: 177-81 (2016) Article DOI: 10.1021/acsmedchemlett.5b00357 BindingDB Entry DOI: 10.7270/Q2Z60QZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50158155 (CHEMBL3781354) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity towards nicotinic acetylcholine receptor in rat P2 brain membranes using [3H]-nicotine as a radioligan... | ACS Med Chem Lett 7: 177-81 (2016) Article DOI: 10.1021/acsmedchemlett.5b00357 BindingDB Entry DOI: 10.7270/Q2Z60QZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50158155 (CHEMBL3781354) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Curated by ChEMBL | Assay Description Inhibition of human recombinant hepsin using H2N(EEdansyl)GKQLRVVNGG (KDabcyl)-NH2 as substrate by fluorometric analysis | ACS Med Chem Lett 7: 177-81 (2016) Article DOI: 10.1021/acsmedchemlett.5b00357 BindingDB Entry DOI: 10.7270/Q2Z60QZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50158155 (CHEMBL3781354) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Curated by ChEMBL | Assay Description Inhibition of human recombinant thrombin using H2N(EEdansyl)GKQLRVVNGG (KDabcyl)-NH2 as substrate by fluorometric analysis | ACS Med Chem Lett 7: 177-81 (2016) Article DOI: 10.1021/acsmedchemlett.5b00357 BindingDB Entry DOI: 10.7270/Q2Z60QZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||