

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50227871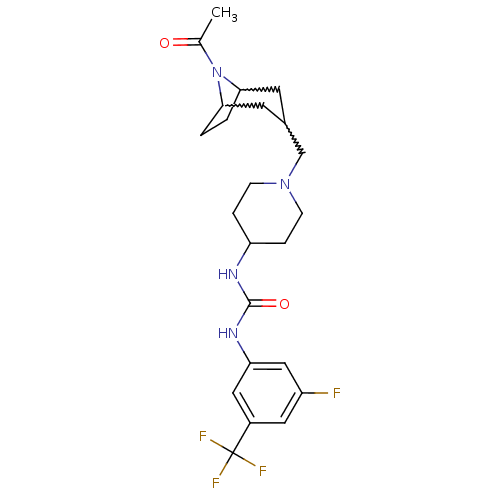 (1-(1-((8-acetyl-8-aza-bicyclo[3.2.1]octan-3-yl)met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 18: 147-51 (2008) Article DOI: 10.1016/j.bmcl.2007.10.109 BindingDB Entry DOI: 10.7270/Q2MC8ZRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Mus musculus) | BDBM50227871 (1-(1-((8-acetyl-8-aza-bicyclo[3.2.1]octan-3-yl)met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery Curated by ChEMBL | Assay Description Antagonist activity at mouse CXCR3 | Bioorg Med Chem Lett 18: 147-51 (2008) Article DOI: 10.1016/j.bmcl.2007.10.109 BindingDB Entry DOI: 10.7270/Q2MC8ZRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||