

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50318159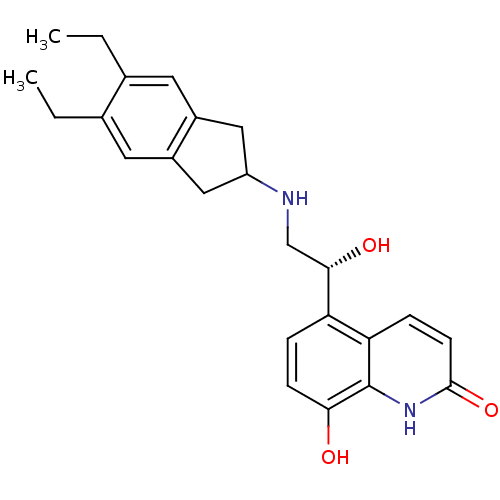 (8-Hydroxy-5-[(R)-1-hydroxy-2-(5,6-diethylindan-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Binding affinity to human beta2-adrenoceptor by radioligand binding assay | Bioorg Med Chem Lett 22: 6280-5 (2012) Article DOI: 10.1016/j.bmcl.2012.07.096 BindingDB Entry DOI: 10.7270/Q2G73G09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50318159 (8-Hydroxy-5-[(R)-1-hydroxy-2-(5,6-diethylindan-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CGP12177 from human beta2 adrenoceptor | Bioorg Med Chem Lett 20: 5302-7 (2010) Article DOI: 10.1016/j.bmcl.2010.06.136 BindingDB Entry DOI: 10.7270/Q2HD7VVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50318159 (8-Hydroxy-5-[(R)-1-hydroxy-2-(5,6-diethylindan-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]iodo-(+/-)-cyanopindolol from human adrenergic beta2 receptor expressed in CHO cells after 3 hrs by radio-ligand binding assay | Bioorg Med Chem Lett 24: 4341-7 (2014) Article DOI: 10.1016/j.bmcl.2014.06.014 BindingDB Entry DOI: 10.7270/Q2Z89F2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50318159 (8-Hydroxy-5-[(R)-1-hydroxy-2-(5,6-diethylindan-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-dihydroalprenolol from human beta2-adrenoceptor expressed in HEK cells after 4 hrs | Bioorg Med Chem Lett 21: 1354-8 (2011) Checked by Author Article DOI: 10.1016/j.bmcl.2011.01.043 BindingDB Entry DOI: 10.7270/Q2NC61HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50318159 (8-Hydroxy-5-[(R)-1-hydroxy-2-(5,6-diethylindan-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assay | J Med Chem 53: 3675-84 (2010) Article DOI: 10.1021/jm100068m BindingDB Entry DOI: 10.7270/Q27H1JRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50318159 (8-Hydroxy-5-[(R)-1-hydroxy-2-(5,6-diethylindan-2-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]iodo-(+/-)-cyanopindolol from human adrenergic beta1 receptor expressed in CHO cells after 3 hrs by radio-ligand binding assay | Bioorg Med Chem Lett 24: 4341-7 (2014) Article DOI: 10.1016/j.bmcl.2014.06.014 BindingDB Entry DOI: 10.7270/Q2Z89F2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50318159 (8-Hydroxy-5-[(R)-1-hydroxy-2-(5,6-diethylindan-2-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CGP12177 from human beta-1 adrenoceptor | Bioorg Med Chem Lett 20: 5302-7 (2010) Article DOI: 10.1016/j.bmcl.2010.06.136 BindingDB Entry DOI: 10.7270/Q2HD7VVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50318159 (8-Hydroxy-5-[(R)-1-hydroxy-2-(5,6-diethylindan-2-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Binding affinity to human beta1-adrenoceptor by radioligand binding assay | Bioorg Med Chem Lett 22: 6280-5 (2012) Article DOI: 10.1016/j.bmcl.2012.07.096 BindingDB Entry DOI: 10.7270/Q2G73G09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50318159 (8-Hydroxy-5-[(R)-1-hydroxy-2-(5,6-diethylindan-2-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]7-HO-PIPAT from human D3R expressed in HEK cells | Bioorg Med Chem Lett 24: 4341-7 (2014) Article DOI: 10.1016/j.bmcl.2014.06.014 BindingDB Entry DOI: 10.7270/Q2Z89F2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50318159 (8-Hydroxy-5-[(R)-1-hydroxy-2-(5,6-diethylindan-2-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||