

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50117592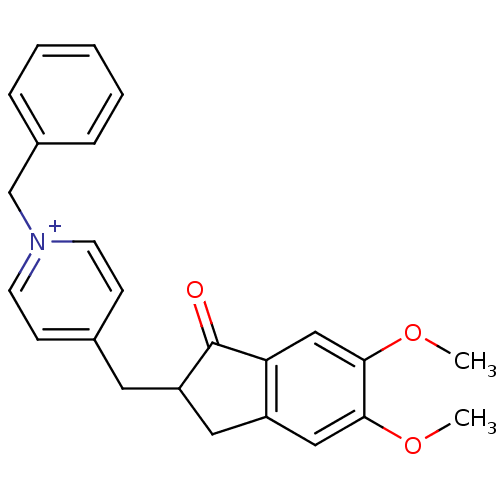 (1-Benzyl-4-(5,6-dimethoxy-1-oxo-indan-2-ylmethyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ... | J Med Chem 60: 5909-5926 (2017) Article DOI: 10.1021/acs.jmedchem.7b00702 BindingDB Entry DOI: 10.7270/Q22V2JC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50117592 (1-Benzyl-4-(5,6-dimethoxy-1-oxo-indan-2-ylmethyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description In vitro reversal of vecuronium-induced neuromuscular block in guinea pig hemi-diaphragm. | Bioorg Med Chem Lett 12: 2565-8 (2002) BindingDB Entry DOI: 10.7270/Q2CZ37Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||