

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50435960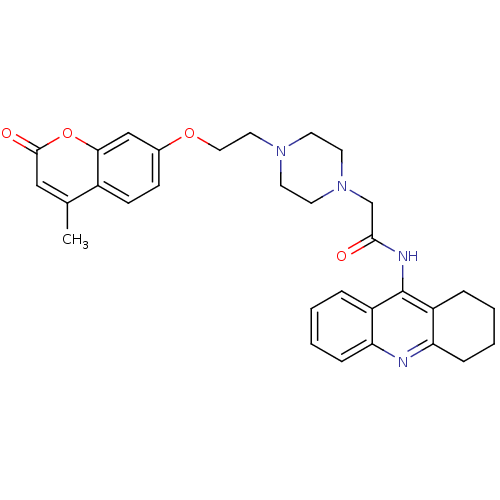 (CHEMBL2391486) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Competitive inhibition of electric eel AChE using acetylthiocholine as substrate by Lineweaver-Burk plot analysis | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50435960 (CHEMBL2391486) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Punjabi University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) | Bioorg Med Chem 22: 3806-14 (2014) Article DOI: 10.1016/j.bmc.2014.05.032 BindingDB Entry DOI: 10.7270/Q2RF5WN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50435960 (CHEMBL2391486) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to 180... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50435960 (CHEMBL2391486) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine as substrate measured for 3.2 mins by Ellman's method | Eur J Med Chem 176: 228-247 (2019) Article DOI: 10.1016/j.ejmech.2019.05.020 BindingDB Entry DOI: 10.7270/Q2KH0RNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||