

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8964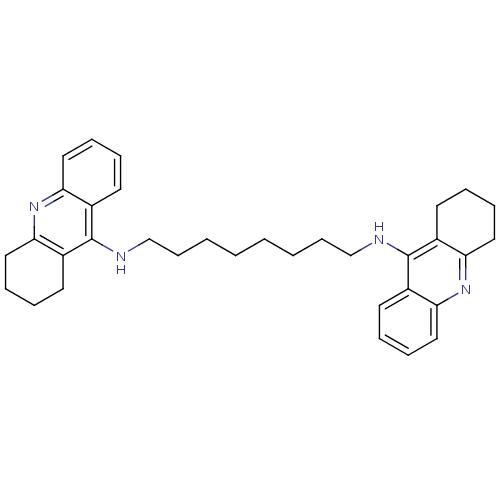 (CHEMBL75274 | Homodimeric Tacrine Analog 3c | N-[8...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | -49.8 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | J Med Chem 48: 1919-29 (2005) Article DOI: 10.1021/jm049510k BindingDB Entry DOI: 10.7270/Q27P8WMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM8964 (CHEMBL75274 | Homodimeric Tacrine Analog 3c | N-[8...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of rat brain Acetylcholinesterase using acetylthiocholine as substrate in presence of BChE inhibitor ethopropazine by spectrophotometric m... | Bioorg Med Chem 27: 895-930 (2019) Article DOI: 10.1016/j.bmc.2019.01.025 BindingDB Entry DOI: 10.7270/Q2N87F3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8964 (CHEMBL75274 | Homodimeric Tacrine Analog 3c | N-[8...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE | Eur J Med Chem 45: 1167-72 (2010) Article DOI: 10.1016/j.ejmech.2009.12.038 BindingDB Entry DOI: 10.7270/Q25H7GFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM8964 (CHEMBL75274 | Homodimeric Tacrine Analog 3c | N-[8...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | 7.4 | 37 |
National Defense Medical Center | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Med Chem 45: 2277-82 (2002) Article DOI: 10.1021/jm010308g BindingDB Entry DOI: 10.7270/Q2KW5D89 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM8964 (CHEMBL75274 | Homodimeric Tacrine Analog 3c | N-[8...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Inhibition of rat brain AChE | J Med Chem 49: 5491-500 (2006) Article DOI: 10.1021/jm060164b BindingDB Entry DOI: 10.7270/Q2154GPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM8964 (CHEMBL75274 | Homodimeric Tacrine Analog 3c | N-[8...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Med Chem 42: 4225-31 (1999) Article DOI: 10.1021/jm990224w BindingDB Entry DOI: 10.7270/Q2W957CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||