

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50150766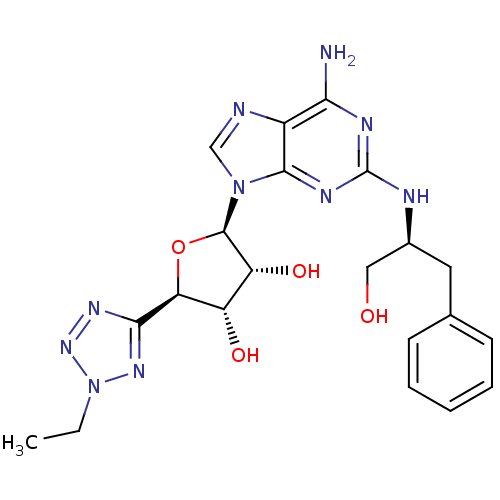 ((2R,3R,4S,5R)-2-(6-amino-2-((S)-1-hydroxy-3-phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]NECA from human recombinant adenosine A2A receptor expressed in Sf21 cells co-expressing GalphaS2, beta4, gamma2 | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50150766 ((2R,3R,4S,5R)-2-(6-amino-2-((S)-1-hydroxy-3-phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara Curated by ChEMBL | Assay Description Displacement of [3H]CGS21680 from human recombinant adenosine A2A receptor expressed in CHO cells after 60 mins by gamma counter | J Med Chem 55: 5676-703 (2012) Article DOI: 10.1021/jm300087j BindingDB Entry DOI: 10.7270/Q25H7HDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50150766 ((2R,3R,4S,5R)-2-(6-amino-2-((S)-1-hydroxy-3-phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IIQAB (CSIC) Curated by ChEMBL | Assay Description Inhibition of [3H]ZM-241385 binding to human adenosine A2A receptor expressed in HeLa cells | J Med Chem 47: 4041-53 (2004) Article DOI: 10.1021/jm031143+ BindingDB Entry DOI: 10.7270/Q26D5TR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50150766 ((2R,3R,4S,5R)-2-(6-amino-2-((S)-1-hydroxy-3-phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human recombinant adenosine receptor A2a | J Med Chem 57: 3623-50 (2014) Article DOI: 10.1021/jm4011669 BindingDB Entry DOI: 10.7270/Q28P621J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50150766 ((2R,3R,4S,5R)-2-(6-amino-2-((S)-1-hydroxy-3-phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at adenosine A2A receptor in human neutrophils assessed as inhibition of fMLP-induced reactive oxygen species release by chemilumine... | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50150766 ((2R,3R,4S,5R)-2-(6-amino-2-((S)-1-hydroxy-3-phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a |
IIQAB (CSIC) Curated by ChEMBL | Assay Description Potency against cAMP formation in CHO cells expressing recombinant human A2A receptor | J Med Chem 47: 4041-53 (2004) Article DOI: 10.1021/jm031143+ BindingDB Entry DOI: 10.7270/Q26D5TR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50150766 ((2R,3R,4S,5R)-2-(6-amino-2-((S)-1-hydroxy-3-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
IIQAB (CSIC) Curated by ChEMBL | Assay Description Efficacy against phenylephrine precontracted tissue relaxation in rat aorta | J Med Chem 47: 4041-53 (2004) Article DOI: 10.1021/jm031143+ BindingDB Entry DOI: 10.7270/Q26D5TR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50150766 ((2R,3R,4S,5R)-2-(6-amino-2-((S)-1-hydroxy-3-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at adenosine receptor A2a in isolated rat aorta assessed as efficacy | J Med Chem 57: 3623-50 (2014) Article DOI: 10.1021/jm4011669 BindingDB Entry DOI: 10.7270/Q28P621J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50150766 ((2R,3R,4S,5R)-2-(6-amino-2-((S)-1-hydroxy-3-phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 9 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at adenosine receptor A2a (unknown origin) assessed as cAMP formation | J Med Chem 57: 3623-50 (2014) Article DOI: 10.1021/jm4011669 BindingDB Entry DOI: 10.7270/Q28P621J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50150766 ((2R,3R,4S,5R)-2-(6-amino-2-((S)-1-hydroxy-3-phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Universit£ di Ferrara Curated by ChEMBL | Assay Description Agonist activity at human recombinant adenosine A2A receptor expressed in CHO cells assessed as induction of cyclic AMP production | J Med Chem 55: 5676-703 (2012) Article DOI: 10.1021/jm300087j BindingDB Entry DOI: 10.7270/Q25H7HDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||