

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50106542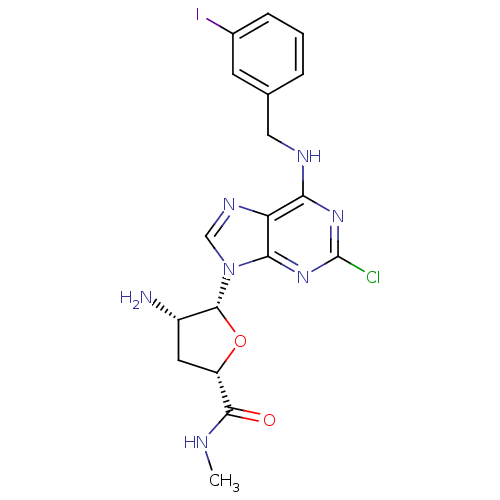 (4-Amino-5-[2-chloro-6-(3-iodo-benzylamino)-purin-9...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity at Mutant (H272E) human adenosine A3 receptor expressed in COS-7 cells | J Med Chem 44: 4125-36 (2001) BindingDB Entry DOI: 10.7270/Q2Z60PSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Rattus norvegicus) | BDBM50106542 (4-Amino-5-[2-chloro-6-(3-iodo-benzylamino)-purin-9...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity at rat adenosine A3 receptor in CHO cells | J Med Chem 44: 4125-36 (2001) BindingDB Entry DOI: 10.7270/Q2Z60PSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50106542 (4-Amino-5-[2-chloro-6-(3-iodo-benzylamino)-purin-9...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity at wild-type Adenosine A3 receptor expressed in COS-7 cells | J Med Chem 44: 4125-36 (2001) BindingDB Entry DOI: 10.7270/Q2Z60PSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Rattus norvegicus) | BDBM50106542 (4-Amino-5-[2-chloro-6-(3-iodo-benzylamino)-purin-9...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Binding affinity against adenosine A3 receptor from rat brain. | J Med Chem 38: 1720-35 (1995) BindingDB Entry DOI: 10.7270/Q21Z453H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||