

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase (Homo sapiens (Human)) | BDBM201239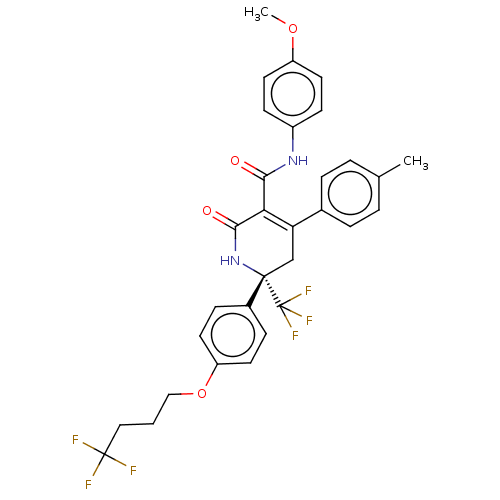 (US9187424, 6-2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The MGAT enzyme reactions were performed in CORNING Falcon 96-well Polypropylene plates, in a total volume of 60 uL of 50 mM Potassium Phosphate buff... | US Patent US9187424 (2015) BindingDB Entry DOI: 10.7270/Q2FQ9VFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase (Homo sapiens (Human)) | BDBM201239 (US9187424, 6-2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant MGAT2 expressed in Sf9 cell membrane using 2-monooleglycerol and [H3]-oleoyl-CoA as substrates incubated for 20 mins ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01356 BindingDB Entry DOI: 10.7270/Q2RF5ZVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase (Rattus norvegicus) | BDBM201239 (US9187424, 6-2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat recombinant MGAT2 expressed in Sf9 cell membrane using 2-oleoylglycerol and oleoyl-CoA as substrates incubated for 10 mins by LCMS ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01356 BindingDB Entry DOI: 10.7270/Q2RF5ZVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase (Mus musculus) | BDBM201239 (US9187424, 6-2) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mouse recombinant MGAT2 expressed in Sf9 cell membrane using 2-oleoylglycerol and oleoyl-CoA as substrates incubated for 10 mins by LCM... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01356 BindingDB Entry DOI: 10.7270/Q2RF5ZVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase (Homo sapiens (Human)) | BDBM201239 (US9187424, 6-2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description MGAT2 enzyme was assayed using membranes isolated from Sf9 cells expressing the recombinant human MGAT2 cDNA with 2-monooleoylglycerol and [3H]-oleoy... | US Patent US9187424 (2015) BindingDB Entry DOI: 10.7270/Q2FQ9VFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||