

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50021153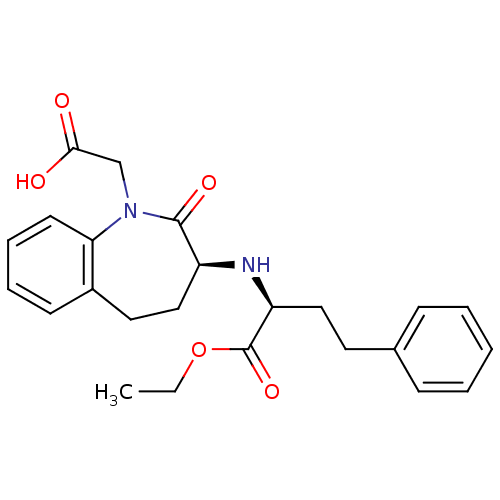 (1H-1-Benzazepine-1-acetic acid, 3-((1-(ethoxycarbo...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 1603-6 (1985) BindingDB Entry DOI: 10.7270/Q2ZK5H7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50021153 (1H-1-Benzazepine-1-acetic acid, 3-((1-(ethoxycarbo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland Curated by ChEMBL | Assay Description Inhibitory activity against angiotensin I converting enzyme (ACE) | J Med Chem 43: 305-41 (2000) Checked by Author BindingDB Entry DOI: 10.7270/Q2JD4XH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021153 (1H-1-Benzazepine-1-acetic acid, 3-((1-(ethoxycarbo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Tested for 50% inhibition of Angiotensin converting enzyme(ACE) obtained from rabbit lung (in vitro) | J Med Chem 37: 1823-32 (1994) BindingDB Entry DOI: 10.7270/Q280537C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021153 (1H-1-Benzazepine-1-acetic acid, 3-((1-(ethoxycarbo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of rabbit lung ACE assessed as hippuryl-histidyl-leucine hydrolysis after 30 mins by colorimetric method | Bioorg Med Chem 23: 3526-33 (2015) Article DOI: 10.1016/j.bmc.2015.04.024 BindingDB Entry DOI: 10.7270/Q2DB83M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021153 (1H-1-Benzazepine-1-acetic acid, 3-((1-(ethoxycarbo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 212 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of rabbit lung ACE assessed as hydrolysis of hippuryl-histidyl-leucine to hippuric acid and histidyl-leucine after 30 mins | Bioorg Med Chem 21: 4485-93 (2013) Article DOI: 10.1016/j.bmc.2013.05.031 BindingDB Entry DOI: 10.7270/Q2P55PXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||