

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aurora kinase A (Homo sapiens (Human)) | BDBM87053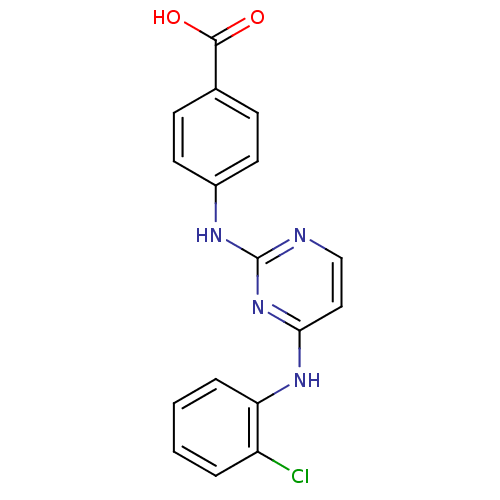 (Bisanilinopyrimidine inhibitor, 7 | Bisanilinopyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2.5 | 15 | n/a | n/a | n/a | 7.4 | 25 |
Moffitt Cancer Center and Research Institute | Assay Description The formation of ADP from ATP was quantified using a coupled enzyme assay (DiscoverX) in with a fluorescent resorufin dye is generated from the inter... | ACS Chem Biol 7: 698-706 (2012) Article DOI: 10.1021/cb200508b BindingDB Entry DOI: 10.7270/Q2PK0DRH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM87053 (Bisanilinopyrimidine inhibitor, 7 | Bisanilinopyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Moffitt Cancer Center | Assay Description In vitro enzyme activity assay using Aurora Kinase A. | J Med Chem 55: 7392-416 (2012) Article DOI: 10.1021/jm300334d BindingDB Entry DOI: 10.7270/Q2V986NH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM87053 (Bisanilinopyrimidine inhibitor, 7 | Bisanilinopyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 2.5 | 15 | n/a | n/a | n/a | 7.4 | n/a |
H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description The binding of inhibitors to Aurora A kinase was analyzed with a MicroCal iTC200 titration calorimeter (GE Healthcare, Piscataway, N.J.). The protein... | US Patent US9249124 (2016) BindingDB Entry DOI: 10.7270/Q2J1020R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM87053 (Bisanilinopyrimidine inhibitor, 7 | Bisanilinopyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | 7.4 | 18 |
H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description Aurora A was exchanged into 50 mM phosphate buffer (pH 7.4) including 1 mM DTT via PD-10 columns and was concentrated to 20 mg mL-1 using Amicon Ultr... | US Patent US9249124 (2016) BindingDB Entry DOI: 10.7270/Q2J1020R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM87053 (Bisanilinopyrimidine inhibitor, 7 | Bisanilinopyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | 7.4 | 25 |
H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description Reactions were carried out at room temperature in 15 mM HEPES buffer (pH 7.4) containing 20 mM NaCl, 1 mM EGTA, 0.02% Tween 20, 10 mM MgCl2, 5% (v/v)... | US Patent US9249124 (2016) BindingDB Entry DOI: 10.7270/Q2J1020R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||