

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aurora kinase B (Homo sapiens (Human)) | BDBM81552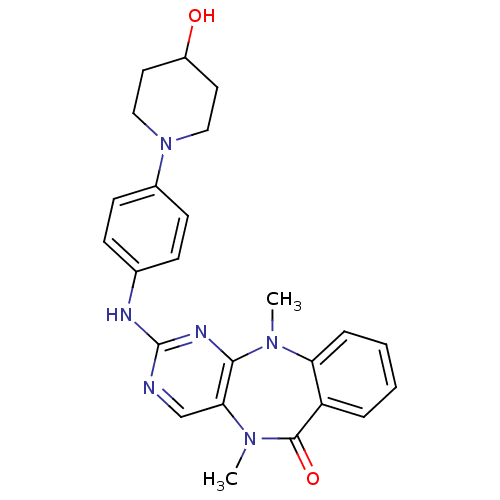 (Benzo[e]pyrimido[5,4-b][1,4]diazepin-6(11H)-one, 1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 18.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School | Assay Description In vitro kinase assay of Aurora A, B, and C using Z-LYTE technology (Invitrogen) and ATP at Km apparent for each kinase. | ACS Chem Biol 7: 185-96 (2012) Article DOI: 10.1021/cb200305u BindingDB Entry DOI: 10.7270/Q2PV6HZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM81552 (Benzo[e]pyrimido[5,4-b][1,4]diazepin-6(11H)-one, 1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School | Assay Description In vitro biochemical assays were performed in parallel to determine the most potent tool compound. | Chem Biol 18: 868-79 (2011) Article DOI: 10.1016/j.chembiol.2011.05.010 BindingDB Entry DOI: 10.7270/Q2HD7T57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||