 Found 3 hits Enz. Inhib. hit(s) with Target = 'Beta-secretase 1' and Ligand = 'BDBM50393103'
Found 3 hits Enz. Inhib. hit(s) with Target = 'Beta-secretase 1' and Ligand = 'BDBM50393103' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50393103
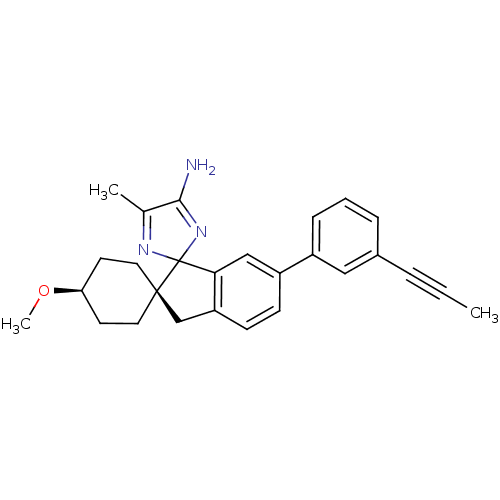
(CHEMBL2151058 | US10231967, Example 20z | US991898...)Show SMILES CO[C@H]1CC[C@@]2(Cc3ccc(cc3C22N=C(C)C(N)=N2)-c2cccc(c2)C#CC)CC1 |r,wD:5.5,2.1,c:20,t:16,(25.81,-10.6,;25.05,-9.26,;23.51,-9.24,;22.75,-7.91,;21.21,-7.89,;20.44,-9.22,;19.54,-10.47,;18.07,-10.01,;16.73,-10.79,;15.4,-10.01,;15.4,-8.47,;16.73,-7.7,;18.06,-8.46,;19.53,-7.98,;18.61,-6.74,;19.5,-5.48,;19,-4.03,;20.97,-5.95,;22.21,-5.03,;20.99,-7.49,;14.07,-7.7,;14.07,-6.16,;12.74,-5.39,;11.4,-6.16,;11.41,-7.71,;12.74,-8.47,;10.08,-8.48,;8.73,-9.24,;7.39,-10,;21.2,-10.56,;22.73,-10.56,)| Show InChI InChI=1S/C27H29N3O/c1-4-6-19-7-5-8-20(15-19)21-9-10-22-17-26(13-11-23(31-3)12-14-26)27(24(22)16-21)29-18(2)25(28)30-27/h5,7-10,15-16,23H,11-14,17H2,1-3H3,(H2,28,30)/t23-,26-,27? | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 9.2 | n/a |
Astrazeneca AB
US Patent
| Assay Description
TR-FRET AssayThe beta-secretase enzyme used in the TR-FRET is prepared as follows: The cDNA for the soluble part of the human beta-Secretase (AA 1-AA... |
US Patent US8865911 (2014)
BindingDB Entry DOI: 10.7270/Q2T72G4T |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50393103

(CHEMBL2151058 | US10231967, Example 20z | US991898...)Show SMILES CO[C@H]1CC[C@@]2(Cc3ccc(cc3C22N=C(C)C(N)=N2)-c2cccc(c2)C#CC)CC1 |r,wD:5.5,2.1,c:20,t:16,(25.81,-10.6,;25.05,-9.26,;23.51,-9.24,;22.75,-7.91,;21.21,-7.89,;20.44,-9.22,;19.54,-10.47,;18.07,-10.01,;16.73,-10.79,;15.4,-10.01,;15.4,-8.47,;16.73,-7.7,;18.06,-8.46,;19.53,-7.98,;18.61,-6.74,;19.5,-5.48,;19,-4.03,;20.97,-5.95,;22.21,-5.03,;20.99,-7.49,;14.07,-7.7,;14.07,-6.16,;12.74,-5.39,;11.4,-6.16,;11.41,-7.71,;12.74,-8.47,;10.08,-8.48,;8.73,-9.24,;7.39,-10,;21.2,-10.56,;22.73,-10.56,)| Show InChI InChI=1S/C27H29N3O/c1-4-6-19-7-5-8-20(15-19)21-9-10-22-17-26(13-11-23(31-3)12-14-26)27(24(22)16-21)29-18(2)25(28)30-27/h5,7-10,15-16,23H,11-14,17H2,1-3H3,(H2,28,30)/t23-,26-,27? | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of BACE by TR-FRET assay |
ACS Med Chem Lett 3: 875-876 (2012)
Article DOI: 10.1021/ml300324q
BindingDB Entry DOI: 10.7270/Q2VM4DC0 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50393103

(CHEMBL2151058 | US10231967, Example 20z | US991898...)Show SMILES CO[C@H]1CC[C@@]2(Cc3ccc(cc3C22N=C(C)C(N)=N2)-c2cccc(c2)C#CC)CC1 |r,wD:5.5,2.1,c:20,t:16,(25.81,-10.6,;25.05,-9.26,;23.51,-9.24,;22.75,-7.91,;21.21,-7.89,;20.44,-9.22,;19.54,-10.47,;18.07,-10.01,;16.73,-10.79,;15.4,-10.01,;15.4,-8.47,;16.73,-7.7,;18.06,-8.46,;19.53,-7.98,;18.61,-6.74,;19.5,-5.48,;19,-4.03,;20.97,-5.95,;22.21,-5.03,;20.99,-7.49,;14.07,-7.7,;14.07,-6.16,;12.74,-5.39,;11.4,-6.16,;11.41,-7.71,;12.74,-8.47,;10.08,-8.48,;8.73,-9.24,;7.39,-10,;21.2,-10.56,;22.73,-10.56,)| Show InChI InChI=1S/C27H29N3O/c1-4-6-19-7-5-8-20(15-19)21-9-10-22-17-26(13-11-23(31-3)12-14-26)27(24(22)16-21)29-18(2)25(28)30-27/h5,7-10,15-16,23H,11-14,17H2,1-3H3,(H2,28,30)/t23-,26-,27? | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of BACE by secreted APPbeta release assay |
ACS Med Chem Lett 3: 875-876 (2012)
Article DOI: 10.1021/ml300324q
BindingDB Entry DOI: 10.7270/Q2VM4DC0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data


