

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM47353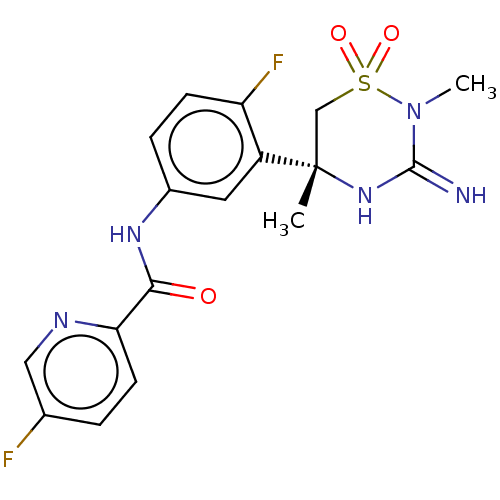 (BDBM143220 | US9029362, 173 | US9687494, 25) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | 0.370 | -54.7 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor IC50s at purified human autoBACE-2 were determined in a time-resolved endpoint proteolysis assay that measures hydrolysis of the QSY7-EISEV... | US Patent US9029362 (2015) BindingDB Entry DOI: 10.7270/Q2X63KP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM47353 (BDBM143220 | US9029362, 173 | US9687494, 25) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | 0.370 | -54.7 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor IC50s at purified human autoBACE-2 were determined in a time-resolved endpoint proteolysis assay that measures hydrolysis of the QSY7-EISEV... | US Patent US8940748 (2015) BindingDB Entry DOI: 10.7270/Q2ZC81MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM47353 (BDBM143220 | US9029362, 173 | US9687494, 25) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | 0.470 | -54.1 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor IC50s at purified human autoBACE-2 were determined in a time-resolved endpoint proteolysis assay that measures hydrolysis of the QSY7-EISEV... | US Patent US9029362 (2015) BindingDB Entry DOI: 10.7270/Q2X63KP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM47353 (BDBM143220 | US9029362, 173 | US9687494, 25) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | 0.470 | -54.1 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor IC50s at purified human autoBACE-2 were determined in a time-resolved endpoint proteolysis assay that measures hydrolysis of the QSY7-EISEV... | US Patent US9029362 (2015) BindingDB Entry DOI: 10.7270/Q2X63KP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM47353 (BDBM143220 | US9029362, 173 | US9687494, 25) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | 370 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description This assay monitored the increase of 620 nm fluorescence that resulted from BACE1 cleavage of an APPswedish APPswe mutant peptide FRET substrate (QSY... | US Patent US9687494 (2017) BindingDB Entry DOI: 10.7270/Q2CJ8BN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM47353 (BDBM143220 | US9029362, 173 | US9687494, 25) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human BACE2 | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM47353 (BDBM143220 | US9029362, 173 | US9687494, 25) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BACE2 (unknown origin) using APP harboring Swedish Lys/Met mutant-derived peptide as substrate incubated for 2 hrs by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01858 BindingDB Entry DOI: 10.7270/Q2GH9NQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||