

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bile acid receptor (Homo sapiens (Human)) | BDBM21725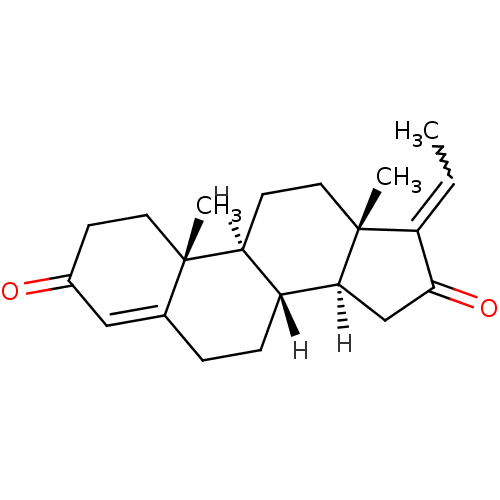 ((1S,2R,10R,11S,14Z,15S)-14-ethylidene-2,15-dimethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Perugia Curated by ChEMBL | Assay Description Inhibitory concentration against Farnesoid X receptor | J Med Chem 48: 6948-55 (2005) Article DOI: 10.1021/jm0505056 BindingDB Entry DOI: 10.7270/Q20V8DKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM21725 ((1S,2R,10R,11S,14Z,15S)-14-ethylidene-2,15-dimethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity against FXR assessed as transactivation of luciferase reporter gene in CV1 cells | Bioorg Med Chem Lett 16: 5398-402 (2006) Article DOI: 10.1016/j.bmcl.2006.07.079 BindingDB Entry DOI: 10.7270/Q2H41S84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM21725 ((1S,2R,10R,11S,14Z,15S)-14-ethylidene-2,15-dimethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
City of Hope National Medical Center Curated by ChEMBL | Assay Description Antagonist activity at FXR (unknown origin) by coactivator assay | Bioorg Med Chem 21: 4266-78 (2013) Article DOI: 10.1016/j.bmc.2013.04.069 BindingDB Entry DOI: 10.7270/Q2J67J9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM21725 ((1S,2R,10R,11S,14Z,15S)-14-ethylidene-2,15-dimethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Antagonist activity at GST-tagged FXRalpha LBD (unknown origin) assessed as inhibition of CDCA-induced bio-SRC-1 recruitment after 30 mins by fluores... | Bioorg Med Chem 22: 1596-607 (2014) Article DOI: 10.1016/j.bmc.2014.01.032 BindingDB Entry DOI: 10.7270/Q29Z96CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM21725 ((1S,2R,10R,11S,14Z,15S)-14-ethylidene-2,15-dimethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Perugia Curated by ChEMBL | Assay Description Inhibitory concentration against Farnesoid X receptor; range from 50-100000 | J Med Chem 48: 6948-55 (2005) Article DOI: 10.1021/jm0505056 BindingDB Entry DOI: 10.7270/Q20V8DKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM21725 ((1S,2R,10R,11S,14Z,15S)-14-ethylidene-2,15-dimethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.20E+4 | n/a | n/a | 7.2 | 37 |
University of Tokyo | Assay Description Compounds were screened for agonist/antagonist activity on FXR-GAL4 chimeric receptors in transiently transfected HEK-293 cells. The EC50/IC50 values... | Bioorg Med Chem 15: 2587-600 (2007) Article DOI: 10.1016/j.bmc.2007.01.046 BindingDB Entry DOI: 10.7270/Q21R6NSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||