

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM222048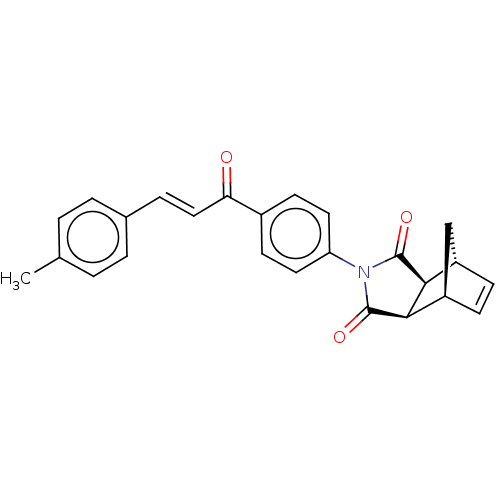 ((3aR,4S,7R,7aS)-2-(4-((E)-3-(p-tolyl)acryloyl)phen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.405 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112923 BindingDB Entry DOI: 10.7270/Q21N852G | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM222048 ((3aR,4S,7R,7aS)-2-(4-((E)-3-(p-tolyl)acryloyl)phen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.605 | -52.6 | 0.597 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Cumhuriyet University | Assay Description Esterase activity assay was performed based on the method of by Verpoorte et al. [Verpoorte et al., J. Biol. Chem., 242:4221-4229] as described in pr... | Bioorg Chem 70: 118-125 (2017) Article DOI: 10.1016/j.bioorg.2016.12.001 BindingDB Entry DOI: 10.7270/Q2CF9NZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||