

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM25900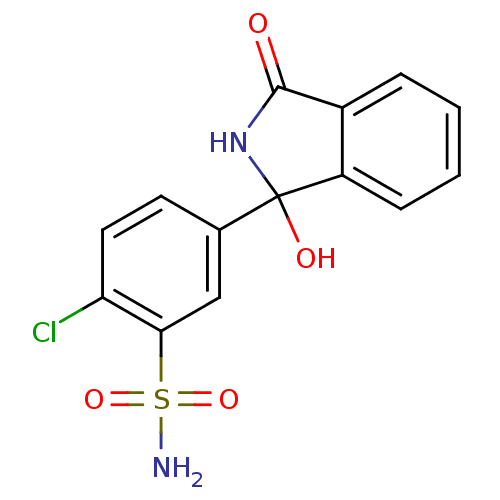 (2-chloro-5-(1-hydroxy-3-oxo-2,3-dihydro-1H-isoindo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents | Article PubMed | 348 | -36.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Universita degli Studi di Firenze | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | Bioorg Med Chem Lett 18: 2567-73 (2008) Article DOI: 10.1016/j.bmcl.2008.03.051 BindingDB Entry DOI: 10.7270/Q23N21P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM25900 (2-chloro-5-(1-hydroxy-3-oxo-2,3-dihydro-1H-isoindo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents | Article PubMed | 3.48E+5 | -19.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Laboratoire Synthèse et Réactivité des Substances Naturelles | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA catalysed CO2 hydration activity [Khalifah et al., J. Biol. Chem., ... | J Enzyme Inhib Med Chem 27: 886-91 (2012) Article DOI: 10.3109/14756366.2011.638921 BindingDB Entry DOI: 10.7270/Q2M0449D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM25900 (2-chloro-5-(1-hydroxy-3-oxo-2,3-dihydro-1H-isoindo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents | US Patent | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Naia Metabolic, Inc. US Patent | Assay Description Compounds which are CAIs are well known in the art, see for example, Pastorekova et al, Journal of Enzyme Inhibition and Medicinal Chemistry, 19(3), ... | US Patent US10172837 (2019) BindingDB Entry DOI: 10.7270/Q2VT1V5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||