 Found 8 hits Enz. Inhib. hit(s) with Target = 'Carbonic anhydrase 1' and Ligand = 'BDBM50079068'
Found 8 hits Enz. Inhib. hit(s) with Target = 'Carbonic anhydrase 1' and Ligand = 'BDBM50079068' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50079068
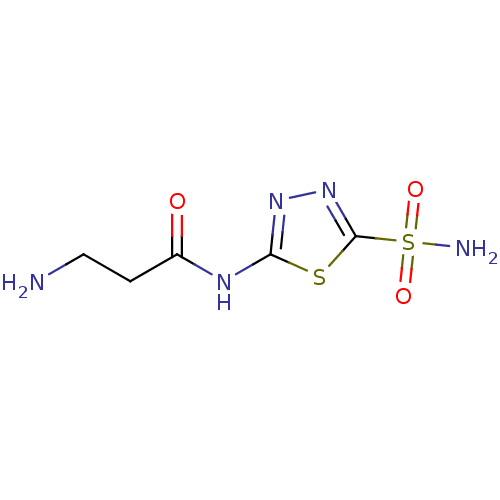
(3-amino-N-(5-sulfamoyl-[1,3,4]thiadiazol-2-yl)-pro...)Show InChI InChI=1S/C5H9N5O3S2/c6-2-1-3(11)8-4-9-10-5(14-4)15(7,12)13/h1-2,6H2,(H2,7,12,13)(H,8,9,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 1 preincubated for 15 mins by CO2 hydration stopped-flow assay |
Bioorg Med Chem 21: 1534-8 (2013)
Article DOI: 10.1016/j.bmc.2012.07.024
BindingDB Entry DOI: 10.7270/Q2VQ3463 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50079068

(3-amino-N-(5-sulfamoyl-[1,3,4]thiadiazol-2-yl)-pro...)Show InChI InChI=1S/C5H9N5O3S2/c6-2-1-3(11)8-4-9-10-5(14-4)15(7,12)13/h1-2,6H2,(H2,7,12,13)(H,8,9,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 454 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
A.P.S. University
Curated by ChEMBL
| Assay Description
Inhibition of human CA1 |
Bioorg Med Chem Lett 16: 2044-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.08.071
BindingDB Entry DOI: 10.7270/Q20Z761V |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50079068

(3-amino-N-(5-sulfamoyl-[1,3,4]thiadiazol-2-yl)-pro...)Show InChI InChI=1S/C5H9N5O3S2/c6-2-1-3(11)8-4-9-10-5(14-4)15(7,12)13/h1-2,6H2,(H2,7,12,13)(H,8,9,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 455 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibition of human cloned Carbonic anhydrase I (hCA I,cytosolic form) |
J Med Chem 42: 2641-50 (1999)
Article DOI: 10.1021/jm9900523
BindingDB Entry DOI: 10.7270/Q2J67HMK |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50079068

(3-amino-N-(5-sulfamoyl-[1,3,4]thiadiazol-2-yl)-pro...)Show InChI InChI=1S/C5H9N5O3S2/c6-2-1-3(11)8-4-9-10-5(14-4)15(7,12)13/h1-2,6H2,(H2,7,12,13)(H,8,9,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 455 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant carbonic anhydrase I (CA1) |
J Med Chem 42: 3690-700 (1999)
Article DOI: 10.1021/jm9901879
BindingDB Entry DOI: 10.7270/Q26Q1XXC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50079068

(3-amino-N-(5-sulfamoyl-[1,3,4]thiadiazol-2-yl)-pro...)Show InChI InChI=1S/C5H9N5O3S2/c6-2-1-3(11)8-4-9-10-5(14-4)15(7,12)13/h1-2,6H2,(H2,7,12,13)(H,8,9,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 455 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase I |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50079068

(3-amino-N-(5-sulfamoyl-[1,3,4]thiadiazol-2-yl)-pro...)Show InChI InChI=1S/C5H9N5O3S2/c6-2-1-3(11)8-4-9-10-5(14-4)15(7,12)13/h1-2,6H2,(H2,7,12,13)(H,8,9,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 455 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase I (CAI) |
Bioorg Med Chem Lett 10: 1117-20 (2000)
BindingDB Entry DOI: 10.7270/Q2WW7J6M |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50079068

(3-amino-N-(5-sulfamoyl-[1,3,4]thiadiazol-2-yl)-pro...)Show InChI InChI=1S/C5H9N5O3S2/c6-2-1-3(11)8-4-9-10-5(14-4)15(7,12)13/h1-2,6H2,(H2,7,12,13)(H,8,9,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 455 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase I (CA1) |
J Med Chem 43: 292-300 (2000)
BindingDB Entry DOI: 10.7270/Q22806T7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50079068

(3-amino-N-(5-sulfamoyl-[1,3,4]thiadiazol-2-yl)-pro...)Show InChI InChI=1S/C5H9N5O3S2/c6-2-1-3(11)8-4-9-10-5(14-4)15(7,12)13/h1-2,6H2,(H2,7,12,13)(H,8,9,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 455 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibition of cloned isozyme, human carbonic anhydrase I |
Bioorg Med Chem Lett 11: 575-82 (2001)
BindingDB Entry DOI: 10.7270/Q2KW5GJQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data