

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM11635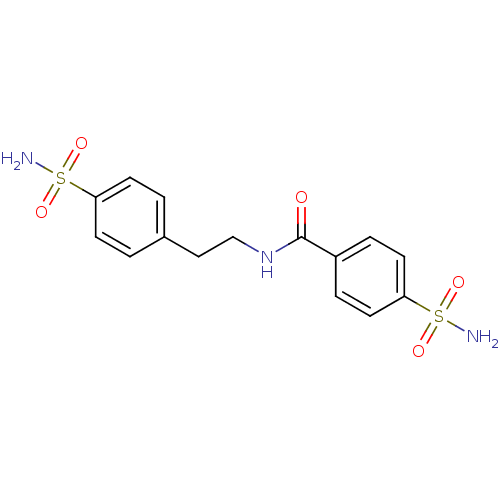 (4-sulfamoyl-N-[2-(4-sulfamoylphenyl)ethyl]benzamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | -46.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Istituto di Biostrutture e Bioimmagini-CNR | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 48: 5721-7 (2005) Article DOI: 10.1021/jm050333c BindingDB Entry DOI: 10.7270/Q2ST7N2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM11635 (4-sulfamoyl-N-[2-(4-sulfamoylphenyl)ethyl]benzamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human carbonic anhydrase II (hCA II) | Bioorg Med Chem Lett 13: 2759-63 (2003) BindingDB Entry DOI: 10.7270/Q2959GZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM11635 (4-sulfamoyl-N-[2-(4-sulfamoylphenyl)ethyl]benzamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitiory activity against human Carbonic anhydrase II (hCA II) | Bioorg Med Chem Lett 15: 4862-6 (2005) Article DOI: 10.1016/j.bmcl.2005.08.048 BindingDB Entry DOI: 10.7270/Q2NK3DMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM11635 (4-sulfamoyl-N-[2-(4-sulfamoylphenyl)ethyl]benzamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Superior Técnico Curated by ChEMBL | Assay Description Inhibition of human recombinant CA2 by stopped-flow CO2 hydrase assay | J Med Chem 51: 7968-79 (2008) Article DOI: 10.1021/jm800964f BindingDB Entry DOI: 10.7270/Q2XK8FDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM11635 (4-sulfamoyl-N-[2-(4-sulfamoylphenyl)ethyl]benzamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Universit£ Montpellier II Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase II by spectrophotometry at pH 7.5 | Bioorg Med Chem 19: 1172-8 (2011) Article DOI: 10.1016/j.bmc.2010.12.048 BindingDB Entry DOI: 10.7270/Q2C53M44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM11635 (4-sulfamoyl-N-[2-(4-sulfamoylphenyl)ethyl]benzamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LNEG Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 2 | Bioorg Med Chem Lett 20: 3623-7 (2010) Article DOI: 10.1016/j.bmcl.2010.04.109 BindingDB Entry DOI: 10.7270/Q2J38TJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM11635 (4-sulfamoyl-N-[2-(4-sulfamoylphenyl)ethyl]benzamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Slovak Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 2 after 15 mins by stopped flow CO2 hydration assay | Bioorg Med Chem 20: 1403-10 (2012) Article DOI: 10.1016/j.bmc.2012.01.007 BindingDB Entry DOI: 10.7270/Q2VH5P89 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM11635 (4-sulfamoyl-N-[2-(4-sulfamoylphenyl)ethyl]benzamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Binding affinity against human carbonic anhydrase II (hCA -II) | Bioorg Med Chem Lett 11: 1787-91 (2001) BindingDB Entry DOI: 10.7270/Q2K64JM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM11635 (4-sulfamoyl-N-[2-(4-sulfamoylphenyl)ethyl]benzamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Compound was evaluated for inhibition against human carbonic anhydrase II | Bioorg Med Chem Lett 14: 217-23 (2003) BindingDB Entry DOI: 10.7270/Q2154HMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM11635 (4-sulfamoyl-N-[2-(4-sulfamoylphenyl)ethyl]benzamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Superior T£cnico Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 2 after 15 mins by stopped flow CO2 hydration assay | Bioorg Med Chem 18: 5081-9 (2010) Article DOI: 10.1016/j.bmc.2010.05.072 BindingDB Entry DOI: 10.7270/Q2T154M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM11635 (4-sulfamoyl-N-[2-(4-sulfamoylphenyl)ethyl]benzamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Kochi Medical School Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 2 at pH 7.5 by stopped flow CO2 hydration assay | Bioorg Med Chem 19: 5023-30 (2011) Article DOI: 10.1016/j.bmc.2011.06.038 BindingDB Entry DOI: 10.7270/Q24T6JRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||