

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50163864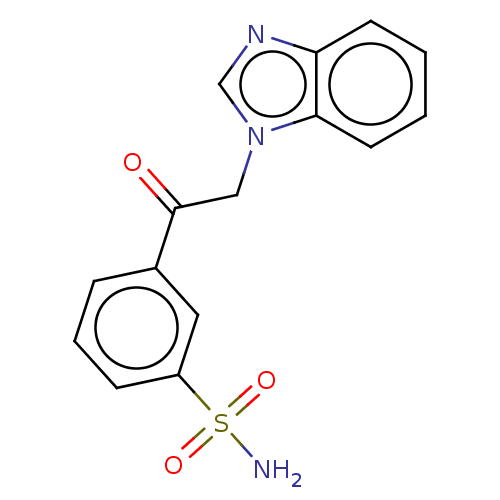 (3-(1H-benzimidazol-1-ylacetyl)benzenesulfonamide (...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 8.20E+5 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA2 expressed in Escherichia coli assessed as association rate constant after 30 secs by surface plasmon resona... | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50163864 (3-(1H-benzimidazol-1-ylacetyl)benzenesulfonamide (...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 4.34E+3 | n/a | n/a | n/a | 7.0 | n/a |
Vilnius University | Assay Description The thermal shift assay (TSA) measurements were performed in a Corbett Rotor-Gene 6000 (QIAGEN Rotor-Gene Q, Sydney, Australia) instrument using the ... | J Enzyme Inhib Med Chem 29: 124-31 (2014) Article DOI: 10.3109/14756366.2012.757223 BindingDB Entry DOI: 10.7270/Q2RV0MMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50163864 (3-(1H-benzimidazol-1-ylacetyl)benzenesulfonamide (...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | 0.620 | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA2 expressed in Escherichia coli assessed as dissociation rate constant after 30 secs by surface plasmon reson... | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50163864 (3-(1H-benzimidazol-1-ylacetyl)benzenesulfonamide (...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a |
Vilnius University Curated by ChEMBL | Assay Description Binding affinity to human full length His-tagged CA2 (1 to 260 residues) expressed in Escherichia coli BL21(DE3) assessed as intrinsic thermodynamic ... | J Med Chem 61: 2292-2302 (2018) Article DOI: 10.1021/acs.jmedchem.7b01408 BindingDB Entry DOI: 10.7270/Q23T9KNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50163864 (3-(1H-benzimidazol-1-ylacetyl)benzenesulfonamide (...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA2 expressed in Escherichia coli by fluorescence-based thermal shift assay | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50163864 (3-(1H-benzimidazol-1-ylacetyl)benzenesulfonamide (...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | 0.620 | n/a | n/a | n/a |
Vilnius University Curated by ChEMBL | Assay Description Binding affinity to human full length His-tagged CA2 (1 to 260 residues) expressed in Escherichia coli BL21(DE3) assessed as dissociation rate consta... | J Med Chem 61: 2292-2302 (2018) Article DOI: 10.1021/acs.jmedchem.7b01408 BindingDB Entry DOI: 10.7270/Q23T9KNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50163864 (3-(1H-benzimidazol-1-ylacetyl)benzenesulfonamide (...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA2 expressed in Escherichia coli after 30 secs by surface plasmon resonance assay | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||