 Found 7 hits Enz. Inhib. hit(s) with Target = 'Carbonic anhydrase 4' and Ligand = 'BDBM13063'
Found 7 hits Enz. Inhib. hit(s) with Target = 'Carbonic anhydrase 4' and Ligand = 'BDBM13063' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase 4
(Bos taurus (bovine)) | BDBM13063
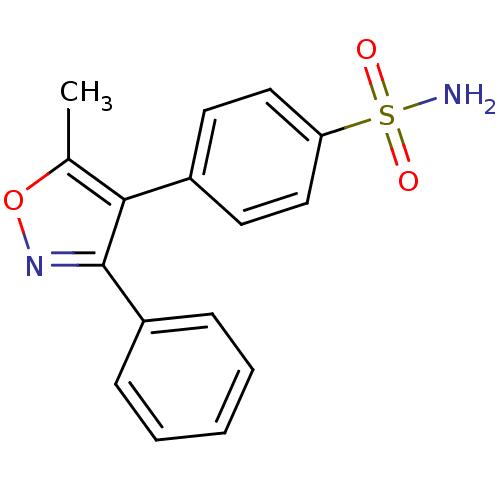
(4-(5-methyl-3-phenyl-1,2-oxazol-4-yl)benzene-1-sul...)Show InChI InChI=1S/C16H14N2O3S/c1-11-15(12-7-9-14(10-8-12)22(17,19)20)16(18-21-11)13-5-3-2-4-6-13/h2-10H,1H3,(H2,17,19,20) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Biostrutture e Bioimmagini-CNR
Curated by ChEMBL
| Assay Description
Inhibitory activity against CA4 isolated from bovine lung microsomes |
Bioorg Med Chem Lett 16: 437-42 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.040
BindingDB Entry DOI: 10.7270/Q25H7H23 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM13063

(4-(5-methyl-3-phenyl-1,2-oxazol-4-yl)benzene-1-sul...)Show InChI InChI=1S/C16H14N2O3S/c1-11-15(12-7-9-14(10-8-12)22(17,19)20)16(18-21-11)13-5-3-2-4-6-13/h2-10H,1H3,(H2,17,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112923
BindingDB Entry DOI: 10.7270/Q21N852G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Bos taurus (bovine)) | BDBM13063

(4-(5-methyl-3-phenyl-1,2-oxazol-4-yl)benzene-1-sul...)Show InChI InChI=1S/C16H14N2O3S/c1-11-15(12-7-9-14(10-8-12)22(17,19)20)16(18-21-11)13-5-3-2-4-6-13/h2-10H,1H3,(H2,17,19,20) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine carbonic anhydrase IV (bCAIV) |
Bioorg Med Chem Lett 15: 1149-54 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.009
BindingDB Entry DOI: 10.7270/Q2JS9R6Q |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM13063

(4-(5-methyl-3-phenyl-1,2-oxazol-4-yl)benzene-1-sul...)Show InChI InChI=1S/C16H14N2O3S/c1-11-15(12-7-9-14(10-8-12)22(17,19)20)16(18-21-11)13-5-3-2-4-6-13/h2-10H,1H3,(H2,17,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase IV (hCAIV) |
Bioorg Med Chem Lett 15: 1149-54 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.009
BindingDB Entry DOI: 10.7270/Q2JS9R6Q |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM13063

(4-(5-methyl-3-phenyl-1,2-oxazol-4-yl)benzene-1-sul...)Show InChI InChI=1S/C16H14N2O3S/c1-11-15(12-7-9-14(10-8-12)22(17,19)20)16(18-21-11)13-5-3-2-4-6-13/h2-10H,1H3,(H2,17,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length CA4 pre-incubated for 15 mins by stopped-flow CO2 hydration method |
J Med Chem 55: 9619-29 (2012)
Article DOI: 10.1021/jm300878g
BindingDB Entry DOI: 10.7270/Q29S1S5G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Bos taurus (bovine)) | BDBM13063

(4-(5-methyl-3-phenyl-1,2-oxazol-4-yl)benzene-1-sul...)Show InChI InChI=1S/C16H14N2O3S/c1-11-15(12-7-9-14(10-8-12)22(17,19)20)16(18-21-11)13-5-3-2-4-6-13/h2-10H,1H3,(H2,17,19,20) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Marburg
| Assay Description
Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... |
J Med Chem 47: 550-7 (2004)
Article DOI: 10.1021/jm030912m
BindingDB Entry DOI: 10.7270/Q2W957DZ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Bos taurus (bovine)) | BDBM13063

(4-(5-methyl-3-phenyl-1,2-oxazol-4-yl)benzene-1-sul...)Show InChI InChI=1S/C16H14N2O3S/c1-11-15(12-7-9-14(10-8-12)22(17,19)20)16(18-21-11)13-5-3-2-4-6-13/h2-10H,1H3,(H2,17,19,20) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Bruker-AXS s.r.l.
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against bovine carbonic anhydrase IV |
Bioorg Med Chem Lett 14: 337-41 (2003)
BindingDB Entry DOI: 10.7270/Q2W66MBZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data