

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM10872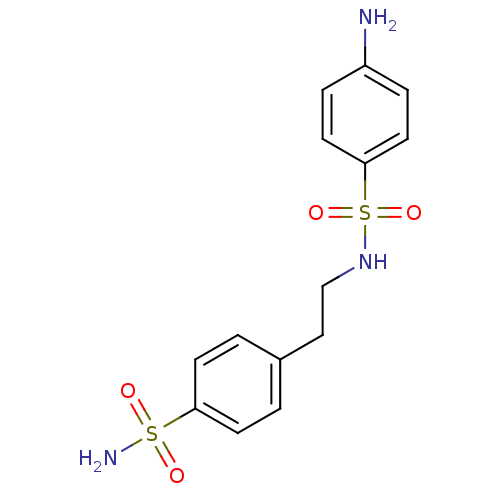 (4-amino-N-[2-(4-sulfamoylphenyl)ethyl]benzene-1-su...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro Curated by ChEMBL | Assay Description Inhibitory constant against human Carbonic anhydrase IX | Bioorg Med Chem Lett 15: 2359-64 (2005) Article DOI: 10.1016/j.bmcl.2005.02.087 BindingDB Entry DOI: 10.7270/Q2ZC82CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM10872 (4-amino-N-[2-(4-sulfamoylphenyl)ethyl]benzene-1-su...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human cloned carbonic anhydrase isozyme IX, by CO2 hydrase assay method. | Bioorg Med Chem Lett 13: 1005-9 (2003) BindingDB Entry DOI: 10.7270/Q2PV6KXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM10872 (4-amino-N-[2-(4-sulfamoylphenyl)ethyl]benzene-1-su...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kochi Medical School Curated by ChEMBL | Assay Description Inhibition of catalytic domain of human recombinant CA IX | J Med Chem 50: 381-8 (2007) Article DOI: 10.1021/jm0612057 BindingDB Entry DOI: 10.7270/Q29G5NNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM10872 (4-amino-N-[2-(4-sulfamoylphenyl)ethyl]benzene-1-su...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tampere Curated by ChEMBL | Assay Description Inhibitory activity against human carbonic anhydrase IX (hCA IX) by using CO2 hydrase assay method | Bioorg Med Chem Lett 14: 3757-62 (2004) Article DOI: 10.1016/j.bmcl.2004.04.106 BindingDB Entry DOI: 10.7270/Q24Q7VJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM10872 (4-amino-N-[2-(4-sulfamoylphenyl)ethyl]benzene-1-su...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kochi Medical School Curated by ChEMBL | Assay Description Inhibitory constant against catalytic domain of human carbonic anhydrase IX | Bioorg Med Chem Lett 15: 3828-33 (2005) Article DOI: 10.1016/j.bmcl.2005.06.055 BindingDB Entry DOI: 10.7270/Q2C82B2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM10872 (4-amino-N-[2-(4-sulfamoylphenyl)ethyl]benzene-1-su...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laxmi Fumigation and Pest Control (P) Curated by ChEMBL | Assay Description Inhibitory activity against human tumor-associated transmembrane carbonic anhydrase IX. | Bioorg Med Chem Lett 14: 3283-90 (2004) Article DOI: 10.1016/j.bmcl.2004.03.099 BindingDB Entry DOI: 10.7270/Q2K074TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||