

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19489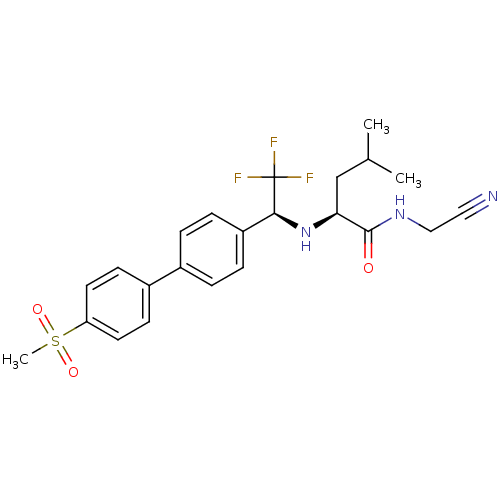 ((2S)-N-(cyanomethyl)-4-methyl-2-[[(1S)-2,2,2-trifl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 17: 4929-33 (2007) Article DOI: 10.1016/j.bmcl.2007.06.023 BindingDB Entry DOI: 10.7270/Q26W98C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19489 ((2S)-N-(cyanomethyl)-4-methyl-2-[[(1S)-2,2,2-trifl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 16: 1985-9 (2006) Article DOI: 10.1016/j.bmcl.2005.12.071 BindingDB Entry DOI: 10.7270/Q237770H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19489 ((2S)-N-(cyanomethyl)-4-methyl-2-[[(1S)-2,2,2-trifl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of humanized rabbit cathepsin K | Bioorg Med Chem Lett 17: 4328-32 (2007) Article DOI: 10.1016/j.bmcl.2007.05.024 BindingDB Entry DOI: 10.7270/Q2TQ618K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19489 ((2S)-N-(cyanomethyl)-4-methyl-2-[[(1S)-2,2,2-trifl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of humanized rabbit cathepsin K | Bioorg Med Chem Lett 18: 923-8 (2008) Article DOI: 10.1016/j.bmcl.2007.12.047 BindingDB Entry DOI: 10.7270/Q21J9BM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19489 ((2S)-N-(cyanomethyl)-4-methyl-2-[[(1S)-2,2,2-trifl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of humanized rabbit cathepsin K | Bioorg Med Chem Lett 21: 920-3 (2011) Article DOI: 10.1016/j.bmcl.2010.12.070 BindingDB Entry DOI: 10.7270/Q2Z89CQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19489 ((2S)-N-(cyanomethyl)-4-methyl-2-[[(1S)-2,2,2-trifl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of human cathepsin K | Bioorg Med Chem Lett 18: 923-8 (2008) Article DOI: 10.1016/j.bmcl.2007.12.047 BindingDB Entry DOI: 10.7270/Q21J9BM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19489 ((2S)-N-(cyanomethyl)-4-methyl-2-[[(1S)-2,2,2-trifl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of cathepsin K (unknown origin) | J Med Chem 51: 4359-69 (2008) Article DOI: 10.1021/jm800219f BindingDB Entry DOI: 10.7270/Q28915N5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19489 ((2S)-N-(cyanomethyl)-4-methyl-2-[[(1S)-2,2,2-trifl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of rabbit cathepsin K | Bioorg Med Chem Lett 18: 923-8 (2008) Article DOI: 10.1016/j.bmcl.2007.12.047 BindingDB Entry DOI: 10.7270/Q21J9BM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||