

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19768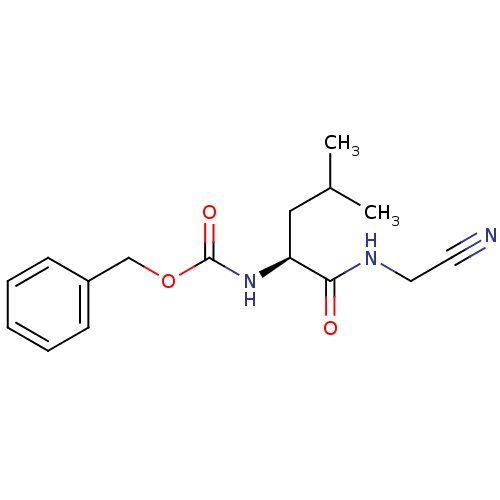 (Cbz-Leu-NH-CH2-CN | JMC487688 Compound 8 | benzyl ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 34.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19768 (Cbz-Leu-NH-CH2-CN | JMC487688 Compound 8 | benzyl ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universitat Bonn | Assay Description Enzyme activities were calculated from kinetic measurements performed by fluorimetric detection of the product AMC at the wavelengths for excitation ... | J Med Chem 48: 7688-707 (2005) Article DOI: 10.1021/jm050686b BindingDB Entry DOI: 10.7270/Q2GB22BG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19768 (Cbz-Leu-NH-CH2-CN | JMC487688 Compound 8 | benzyl ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||