

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19858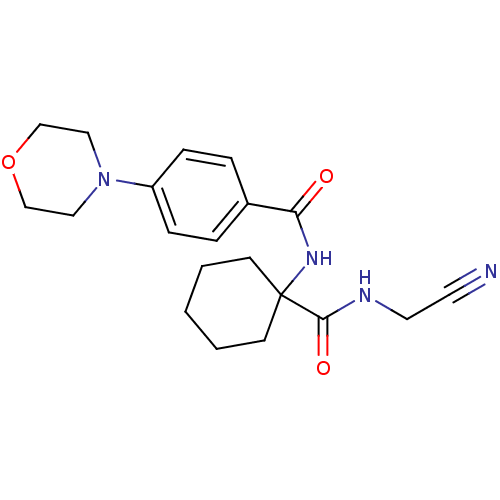 (N-{1-[(cyanomethyl)carbamoyl]cyclohexyl}-4-(morpho...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19858 (N-{1-[(cyanomethyl)carbamoyl]cyclohexyl}-4-(morpho...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 48: 7535-43 (2005) Article DOI: 10.1021/jm0504961 BindingDB Entry DOI: 10.7270/Q28S4N63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19858 (N-{1-[(cyanomethyl)carbamoyl]cyclohexyl}-4-(morpho...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 72 | n/a | 580 | n/a | n/a | 5.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 48: 7535-43 (2005) Article DOI: 10.1021/jm0504961 BindingDB Entry DOI: 10.7270/Q28S4N63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||