

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Homo sapiens (Human)) | BDBM50235800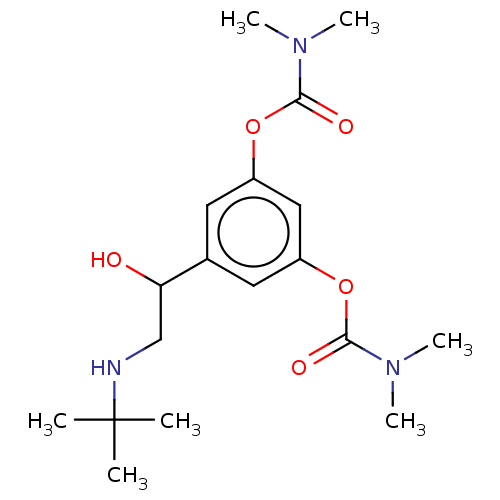 (Bambuterol | CHEBI:553827) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of BuChE (unknown origin) | Eur J Med Chem 132: 294-309 (2017) Article DOI: 10.1016/j.ejmech.2017.03.062 BindingDB Entry DOI: 10.7270/Q2HX1G33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50235800 (Bambuterol | CHEBI:553827) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
South China University of Technology Curated by ChEMBL | Assay Description Inhibitory concentration against cyclin dependent kinase-2 (CDK2)/Cyclin E | Eur J Med Chem 126: 61-71 (2017) Article DOI: 10.1016/j.ejmech.2016.08.061 BindingDB Entry DOI: 10.7270/Q2571F8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50235800 (Bambuterol | CHEBI:553827) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128602 BindingDB Entry DOI: 10.7270/Q2HM5DF5 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50235800 (Bambuterol | CHEBI:553827) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
South China University of Technology Curated by ChEMBL | Assay Description Inhibition of human plasma BuChE using S-butyrylthiocholine iodide as substrate preincubated for 60 mins followed by substrate addition measured afte... | Eur J Med Chem 126: 61-71 (2017) Article DOI: 10.1016/j.ejmech.2016.08.061 BindingDB Entry DOI: 10.7270/Q2571F8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50235800 (Bambuterol | CHEBI:553827) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 119 | n/a | n/a | n/a | n/a | n/a | n/a |
South China University of Technology Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 60 mins followed by substrate addition measured afte... | Eur J Med Chem 126: 61-71 (2017) Article DOI: 10.1016/j.ejmech.2016.08.061 BindingDB Entry DOI: 10.7270/Q2571F8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50235800 (Bambuterol | CHEBI:553827) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 1.30E+5 | n/a | n/a |
South China University of Technology Curated by ChEMBL | Assay Description Antagonism of batrachotoxin-mediated sodium ion uptake into cultured neuroblastoma cells | Eur J Med Chem 126: 61-71 (2017) Article DOI: 10.1016/j.ejmech.2016.08.061 BindingDB Entry DOI: 10.7270/Q2571F8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50235800 (Bambuterol | CHEBI:553827) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 2.40E+3 | n/a | n/a |
South China University of Technology Curated by ChEMBL | Assay Description Antagonism of batrachotoxin-mediated sodium ion uptake into cultured neuroblastoma cells | Eur J Med Chem 126: 61-71 (2017) Article DOI: 10.1016/j.ejmech.2016.08.061 BindingDB Entry DOI: 10.7270/Q2571F8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||