

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Homo sapiens (Human)) | BDBM17277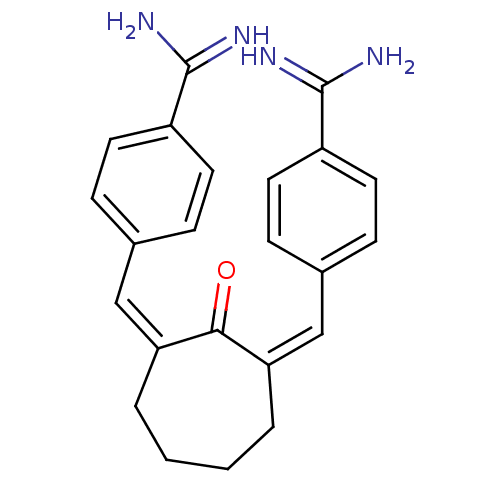 ((Z,Z)BABCH | 4-{[(1Z,3Z)-3-[(4-carbamimidoylphenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.660 | -51.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | Acta Crystallogr D Biol Crystallogr 55: 1395-404 (1999) Article DOI: 10.1107/s0907444999007350 BindingDB Entry DOI: 10.7270/Q2H1308B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17277 ((Z,Z)BABCH | 4-{[(1Z,3Z)-3-[(4-carbamimidoylphenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences Curated by ChEMBL | Assay Description Compound (isomer) was tested in the absence of light for inhibitory activity against Human Coagulation factor X | J Med Chem 41: 3551-6 (1998) Article DOI: 10.1021/jm980281+ BindingDB Entry DOI: 10.7270/Q2QJ7GFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17277 ((Z,Z)BABCH | 4-{[(1Z,3Z)-3-[(4-carbamimidoylphenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences Curated by ChEMBL | Assay Description Inhibitory potency was measured against human coagulation factor X | J Med Chem 41: 3557-62 (1998) Article DOI: 10.1021/jm980280h BindingDB Entry DOI: 10.7270/Q2KS6QPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17277 ((Z,Z)BABCH | 4-{[(1Z,3Z)-3-[(4-carbamimidoylphenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences Curated by ChEMBL | Assay Description Inhibitory activity against human Coagulation factor X | Bioorg Med Chem Lett 10: 957-61 (2000) BindingDB Entry DOI: 10.7270/Q2MG7NRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17277 ((Z,Z)BABCH | 4-{[(1Z,3Z)-3-[(4-carbamimidoylphenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against human Coagulation factor X | Bioorg Med Chem Lett 8: 2235-40 (1999) BindingDB Entry DOI: 10.7270/Q2G44PGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17277 ((Z,Z)BABCH | 4-{[(1Z,3Z)-3-[(4-carbamimidoylphenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences Curated by ChEMBL | Assay Description In vitro inhibition of human coagulation factor Xa (Xa) in a purified enzyme system. | J Med Chem 42: 5415-25 (2000) BindingDB Entry DOI: 10.7270/Q2TT4Q5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17277 ((Z,Z)BABCH | 4-{[(1Z,3Z)-3-[(4-carbamimidoylphenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated against Coagulation factor X | Bioorg Med Chem Lett 8: 1877-82 (1999) BindingDB Entry DOI: 10.7270/Q2222SX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||